Abstract
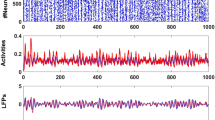
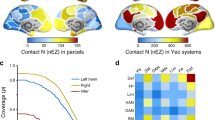
Recurrent networks of dynamic elements frequently exhibit emergent collective oscillations, which can show substantial regularity even when the individual elements are considerably noisy. How noise-induced dynamics at the local level coexists with regular oscillations at the global level is still unclear. Here we show that a combination of stochastic recurrence-based initiation with deterministic refractoriness in an excitable network can reconcile these two features, leading to maximum collective coherence for an intermediate noise level. We report this behaviour in the slow oscillation regime exhibited by a cerebral cortex network under dynamical conditions resembling slow-wave sleep and anaesthesia. Computational analysis of a biologically realistic network model reveals that an intermediate level of background noise leads to quasi-regular dynamics. We verify this prediction experimentally in cortical slices subject to varying amounts of extracellular potassium, which modulates neuronal excitability and thus synaptic noise. The model also predicts that this effectively regular state should exhibit noise-induced memory of the spatial propagation profile of the collective oscillations, which is also verified experimentally. Taken together, these results allow us to construe the high regularity observed experimentally in the brain as an instance of collective stochastic coherence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Enright, J. T. Temporal precision in circadian systems: a reliable neuronal clock from unreliable components? Science 209, 1542–1545 (1980).
Garcia-Ojalvo, J., Elowitz, M. B. & Strogatz, S. H. Modeling a synthetic multicellular clock: repressilators coupled by quorum sensing. Proc. Natl Acad. Sci. USA 101, 10955–10960 (2004).
Sanchez-Vives, M. V. & Mattia, M. Slow wave activity as the default mode of the cerebral cortex. Arch. Ital. Biol. 152, 147–155 (2014).
Steriade, M., Nuñez, A. & Amzica, F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J. Neurosci. 13, 3252–3265 (1993).
Stern, E. A., Kincaid, A. E. & Wilson, C. J. Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal and striatal neurons in vivo. J. Neurophysiol. 77, 1697–1715 (1997).
Shu, Y., Hasenstaub, A. & McCormick, D. A. Turning on and off recurrent balanced cortical activity. Nature 423, 288–293 (2003).
Sanchez-Vives, M. V. & McCormick, D. A. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nature Neurosci. 3, 1027–1034 (2000).
Compte, A. et al. Spontaneous high-frequency (10–80 Hz) oscillations during up states in the cerebral cortex in vitro. J. Neurosci. 28, 13828–13844 (2008).
Mattia, M. & Sanchez-Vives, M. V. Exploring the spectrum of dynamical regimes and timescales in spontaneous cortical activity. Cogn. Neurodynam. 6, 239–250 (2012).
Gluckman, B. J. et al. Stochastic resonance in a neuronal network from mammalian brain. Phys. Rev. Lett. 77, 4098–4101 (1996).
Deco, G., Martí, D., Ledberg, A., Reig, R. & Sanchez-Vives, M. V. Effective reduced diffusion-models: a data driven approach to the analysis of neuronal dynamics. PLoS Comput. Biol. 5, e1000587 (2009).
Steriade, M., Timofeev, I. & Grenier, F. Natural waking and sleep states: a view from inside neocortical neurons. J. Neurophysiol. 85, 1969–1985 (2001).
Parga, N. & Abbott, L. F. Network model of spontaneous activity exhibiting synchronous transitions between up and down states. Front. Neurosci. 1, 57–66 (2007).
Bazhenov, M., Timofeev, I., Steriade, M. & Sejnowski, T. J. Model of thalamocortical slow-wave sleep oscillations and transitions to activated states. J. Neurosci. 22, 8691–8704 (2002).
Pikovsky, A. S. & Kurths, J. Coherence resonance in a noise-driven excitable system. Phys. Rev. Lett. 78, 775–778 (1997).
Lindner, B., Garcia-Ojalvo, J., Neiman, A. & Schimansky-Geier, L. Effects of noise in excitable systems. Phys. Rep. 392, 321–424 (2004).
Compte, A., Sanchez-Vives, M. V., McCormick, D. A. & Wang, X.-J. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J. Neurophysiol. 89, 2707–2725 (2003).
Holcman, D. & Tsodyks, M. The emergence of up and down states in cortical networks. PLoS Comput. Biol. 2, e23 (2006).
Mejias, J. F., Kappen, H. J. & Torres, J. J. Irregular dynamics in up and down cortical states. PLoS ONE 5, e13651 (2010).
Amzica, F., Massimini, M. & Manfridi, A. Spatial buffering during slow and paroxysmal sleep oscillations in cortical networks of glial cells in vivo. J. Neurosci. 22, 1042–1053 (2002).
McCormick, D. A. Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 12, 215–221 (1989).
Romcy-Pereira, R. N., Leite, J. P. & Garcia-Cairasco, N. Synaptic plasticity along the sleep-wake cycle: implications for epilepsy. Epilepsy Behav. 14, 47–53 (2009).
Han, S. K., Yim, T. G., Postnov, D. E. & Sosnovtseva, O. V. Interacting coherence resonance oscillators. Phys. Rev. Lett. 83, 1771–1774 (1999).
Balenzuela, P. & García-Ojalvo, J. Role of chemical synapses in coupled neurons with noise. Phys. Rev. E 72, 021901 (2005).
Bhattacharjee, A., von Hehn, C., Mei, X. & Kaczmarek, L. Localization of the Na-activated K channel slick in the rat central nervous system. J. Comp. Neurol. 484, 80–92 (2005).
Wang, X.-J., Liu, Y., Sanchez-Vives, M. V. & McCormick, D. A. Adaptation and temporal decorrelation by single neurons in the primary visual cortex. J. Neurophysiol. 89, 3279–3293 (2003).
Sanchez-Vives, M. V., Nowak, L. G. & McCormick, D. A. Cellular mechanisms of long-lasting adaptation in visual cortical neurons in vitro. J. Neurosci. 20, 4286–4299 (2000).
Sanchez-Vives, M. V. et al. Inhibitory modulation of cortical up states. J. Neurophysiol. 104, 1314–1324 (2010).
Mattia, M. & Del Giudice, P. Population dynamics of interacting spiking neurons. Phys. Rev. E 66, 051917 (2002).
Satoh, T., Watabe, K. & Eguchi, K. Enhancement during REM sleep of extracellular potassium ion activity in the reticular formation. Brain Res. 174, 180–183 (1979).
Cossart, R., Aronov, D. & Yuste, R. Attractor dynamics of network up states in the neocortex. Nature 423, 283–288 (2003).
Yamaguchi, T. Cerebral extracellular potassium concentration change and cerebral impedance change in short-term ischemia in gerbil. Bull. Tokyo Med. Dental Univ. 33, 1–8 (1986).
Bazhenov, M., Timofeev, I., Steriade, M. & Sejnowski, T. J. Potassium model for slow (2–3 hz) in vivo neocortical paroxysmal oscillations. J. Neurophysiol. 92, 1116–1132 (2004).
Chialvo, D., Cecchi, G. & Magnasco, M. Noise-induced memory in extended excitable systems. Phys. Rev. E 61, 5654–5657 (2000).
Baayen, R. H., Davidson, D. J. & Bates, D. M. Mixed-effects modeling with crossed random effects for subjects and items. J. Mem. Lang. 59, 390–412 (2008).
Sagués, F., Sancho, J. & García-Ojalvo, J. Spatiotemporal order out of noise. Rev. Mod. Phys. 79, 829–882 (2007).
Gang, H., Ditzinger, T., Ning, C. & Haken, H. Stochastic resonance without external periodic force. Phys. Rev. Lett. 71, 807–810 (1993).
Bulsara, A., Jacobs, E., Zhou, T., Moss, F. & Kiss, L. Stochastic resonance in a single neuron model. J. Theor. Biol. 152, 531–555 (1991).
Longtin, A. Stochastic resonance in neuron models. J. Stat. Phys. 70, 309–327 (1993).
Douglass, J. K., Wilkens, L., Pantazelou, E. & Moss, F. Noise enhancement of information transfer in crayfish mechanoreceptors by stochastic resonance. Nature 365, 337–340 (1993).
Levin, J. E. & Miller, J. P. Broadband neural encoding in the cricket cereal sensory system enhanced by stochastic resonance. Nature 380, 165–168 (1996).
Collins, J. J., Imhoff, T. T. & Grigg, P. Noise-enhanced information transmission in rat SA1 cutaneous mechanoreceptors via aperiodic stochastic resonance. J. Neurophysiol. 76, 642–645 (1996).
Stacey, W. C. & Durand, D. M. Stochastic resonance improves signal detection in hippocampal ca1 neurons. J. Neurophysiol. 83, 1394–1402 (2000).
McDonnell, M. D. & Ward, L. M. The benefits of noise in neural systems: bridging theory and experiment. Nature Rev. Neurosci. 12, 415–426 (2011).
Gu, H., Yang, M., Li, L., Liu, Z. & Ren, W. Experimental observation of the stochastic bursting caused by coherence resonance in a neural pacemaker. Neuro Rep. 13, 1657–1660 (2002).
Manjarrez, E. et al. Internal stochastic resonance in the coherence between spinal and cortical neuronal ensembles in the cat. Neurosci. Lett. 326, 93–96 (2002).
Fries, P. Rhythms for cognition: communication through coherence. Neuron 88, 220–235 (2015).
Barardi, A., Sancristóbal, B. & Garcia-Ojalvo, J. Phase-coherence transitions and communication in the gamma range between delay-coupled neuronal populations. PLoS Comput. Biol. 10, e1003723 (2014).
Horikawa, Y. Coherence resonance with multiple peaks in a coupled FitzHugh-Nagumo model. Phys. Rev. E 64, 031905 (2001).
Mountcastle, V. B. Perceptual Neuroscience: The Cerebral Cortex (Harvard Univ. Press, 1998).
Mazzoni, A., Panzeri, S., Logothetis, N. K. & Brunel, N. Encoding of naturalistic stimuli by local field potential spectra in networks of excitatory and inhibitory neurons. PLoS Comput. Biol. 4, e1000239 (2008).
Berens, P., Logothetis, N. K. & Tolias, A. S. Local field potentials, BOLD and spiking activity–relationships and physiological mechanisms. Available at http://precedings.nature.com/documents/5216/version/1 (2010).
Buzsáki, G., Anastassiou, C. A. & Koch, C. The origin of extracellular fields and currents - EEG, ECoG, LFP and spikes. Nature Rev. Neurosci. 13, 407–420 (2012).
Reig, R., Mattia, M., Compte, A., Belmonte, C. & Sanchez-Vives, M. V. Temperature modulation of slow and fast cortical rhythms. J. Neurophysiol. 103, 1253–1261 (2010).
Acknowledgements
We thank A. Compte for useful comments, and our colleagues from CSIC-CNM in Barcelona (X. Villa, R. Villa, G. Gabriel) for providing the recording arrays used in Fig. 5. This work was supported by the Ministerio de Economia y Competividad and FEDER (Spain, projects FIS2012-37655-C02-01, to J.G.-O., and BFU2014-52467-R, to M.V.S.-V.) and EU project CORTICONIC (contract number 600806, to M.V.S.-V.). B.R. was supported by the FPI programme associated to BFU2011-27094 (Spain, Ministerio de Economia y Competividad). J.G.-O. acknowledges support from the ICREA Academia programme and from the Generalitat de Catalunya (project 2014SGR0974).
Author information
Authors and Affiliations
Contributions
B.S., M.V.S.-V. and J.G.-O. conceived the research. B.S. implemented the mathematical model. B.S. and P.B. analysed the data. B.R. performed the experiments. M.V.S.-V. and J.G.-O. supervised the work. B.S., M.V.S.-V. and J.G.-O. wrote the manuscript. All authors revised and approved the text.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 727 kb)
Rights and permissions
About this article
Cite this article
Sancristóbal, B., Rebollo, B., Boada, P. et al. Collective stochastic coherence in recurrent neuronal networks. Nature Phys 12, 881–887 (2016). https://doi.org/10.1038/nphys3739
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphys3739
This article is cited by
-
Altered Neocortical Dynamics in a Mouse Model of Williams–Beuren Syndrome
Molecular Neurobiology (2020)
-
Forecasting Events in the Complex Dynamics of a Semiconductor Laser with Optical Feedback
Scientific Reports (2018)
-
An ultra-compact integrated system for brain activity recording and stimulation validated over cortical slow oscillations in vivo and in vitro
Scientific Reports (2018)
-
Control of in vivo ictogenesis via endogenous synaptic pathways
Scientific Reports (2017)