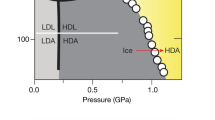
Abstract
The well-known expansion of water on cooling below 277 K is one of several peculiar properties that could signal a second critical point near 220 K and 0.1 GPa in pressure, deep in the supercooled liquid phase. Evidence for this would be a first-order transition line between two distinct supercooled liquids at temperatures below the critical point. As that lies below the minimum crystallization temperature, experimental tests have instead used low- and high-density amorphous ices—LDA and HDA—as proxies for the supercooled liquids. But numerous studies over the past decade have not yielded a clear consensus about the nature of the HDA/LDA transition. Here we identify a previously uncharacterized state of high-density amorphous ice obtained if HDA is annealed at pressures near 2 kbar. The transition between this annealed HDA and LDA is strikingly different from the behaviour found in earlier work, in a way that favours the two-liquid model.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mishima, O. & Stanley, H. E. The relationship between liquid, supercooled and glassy water. Nature 396, 329–335 (1998).
Debenedetti, P. G. Supercooled and glassy water. J. Phys. Condens. Matter 15, R1669–R1726 (2003).
Poole, P. H., Sciortino, F., Essmann, U. & Stanley, H. E. Phase-behaviour of metastable water. Nature 360, 324–328 (1992).
Debenedetti, P. G. & Stanley, H. E. Supercooled and glassy water. Phys. Today 56, 40–46 (2003).
Mishima, O. Reversible first-order transition between two H2O amorphs at ∼0.2 GPa and ∼135 K . J. Chem. Phys. 100, 5910–5912 (1994).
Whalley, E., Klug, D. D. & Handa, Y. P. Entropy of amorphous ice. Nature 342, 782–783 (1989).
Koza, M. M., Schober, H., Fischer, H. E., Hansen, T. & Fujara, F. Kinetics of the high- to low-density amorphous water transition. J. Phys. Condens. Matter 15, 321–332 (2003).
Koza, M. M. et al. Nature of amorphous polymorphism of water. Phys. Rev. Lett. 94, 125506 (2005).
Tulk, C. A. et al. Structural studies of several distinct metastable forms of amorphous ice. Science 297, 1320–1323 (2002).
Guthrie, M. et al. Direct structural measurements of relaxation processes during transformations in amorphous ice. Phys. Rev. B 68, 184110 (2003).
Mishima, O. Relationship between melting and amorphization of ice. Nature 384, 546–549 (1996).
Mishima, O. & Suzuki, Y. Propagation of the polyamorphic transition of ice and the liquid–liquid critical point. Nature 419, 599–603 (2002).
Guthrie, M., Tulk, C. A., Benmore, C. J. & Klug, D. D. A structural study of very high-density amorphous ice. Chem. Phys. Lett. 397, 335–339 (2004).
Tse, J. S. et al. Investigation of the intermediate- and high-density forms of amorphous ice by molecular dynamics calculations and diffraction experiments. Phys. Rev. B 71, 214107 (2005).
Loerting, T., Salzmann, C., Kohl, I., Mayer, E. & Hallbrucker, A. A second distinct structural “state” of high-density amorphous ice at 77 K and 1 bar. Phys. Chem. Chem. Phys. 3, 5355–5357 (2001).
Klug, D. D. Dense ice in detail. Nature 420, 749–751 (2002).
Giovambattista, N., Stanley, H. E. & Sciortino, F. Relation between the high density phase and the very-high density phase of amorphous solid water. Phys. Rev. Lett. 94, 107803 (2005).
Klotz, S. et al. Nature of the polyamorphic transition in ice under pressure. Phys. Rev. Lett. 94, 025506 (2005).
Klotz, S. et al. Reply to Comment on ‘Nature of the polyamorphic transition in ice under pressure’. Phys. Rev. Lett. 96, 149602 (2006).
Besson, J. M. et al. Neutron powder diffraction above 10 GPa. Physica B 180–181, 907–910 (1992).
Bosio, L., Johari, G. P. & Teixeira, J. X-ray study of high-density amorphous water. Phys. Rev. Lett. 56, 460–463 (1986).
Floriano, M. A., Whalley, E., Svensson, E. C. & Sears, V. F. Structure of high-density amorphous ice by neutron-diffraction. Phys. Rev. Lett. 57, 3062–3064 (1986).
Mishima, O., Calvert, L. D. & Whalley, E. Melting ice-I at 77 K and 10 kbar—a new method of making amorphous solids. Nature 310, 393–395 (1984).
Martoňák, R., Donadio, D. & Parrinello, M. Polyamorphism of ice at low temperatures from constant-pressure simulations. Phys. Rev. Lett. 92, 225702 (2004).
Loerting, T. et al. Amorphous ice: Stepwise formation of very-high-density amorphous ice from low-density amorphous ice at 125 K. Phys. Rev. Lett. 96, 025702 (2006).
Andersson, O. Relaxation time of water’s high-density amorphous ice phase. Phys. Rev. Lett. 95, 205503 (2005).
McMillan, P. F., Wilson, M., Daisenberger, D. & Machon, D. A density-driven phase transition between semiconducting and metallic polyamorphs of silicon. Nature Mater. 4, 680–684 (2005).
Besson, J. M. et al. Structural instability in ice VIII under pressure. Phys. Rev. Lett. 78, 3141–3144 (1997).
Acknowledgements
We thank G. Rousse for assistance with preliminary experiments, O. Mishima, D. D. Klug and M. M. Koza for supplementary information and comment, I. Kohl and T. Loerting for additional information about work in ref. 15, and A. M. Saitta, C. Salzmann, K. D. Refson, A. K. Soper and W. C. K. Poon for discussions. We acknowledge support from the UK Engineering and Physical Sciences Research Council, the ISIS Facility at the Rutherford Appleton Laboratory, the Swiss National Science Foundation and the Commission of the European Union (T.S.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Nelmes, R., Loveday, J., Strässle, T. et al. Annealed high-density amorphous ice under pressure. Nature Phys 2, 414–418 (2006). https://doi.org/10.1038/nphys313
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphys313
This article is cited by
-
Observing growth and interfacial dynamics of nanocrystalline ice in thin amorphous ice films
Nature Communications (2024)
-
Absence of amorphous forms when ice is compressed at low temperature
Nature (2019)
-
Hierarchical densification and negative thermal expansion in Ce-based metallic glass under high pressure
Nature Communications (2015)
-
Interplay of the Glass Transition and the Liquid-Liquid Phase Transition in Water
Scientific Reports (2012)
-
The preparation and structure of salty ice VII under pressure
Nature Materials (2009)