Abstract
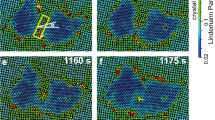
When a system undergoes a transition from a liquid to a solid phase, it passes through multiple intermediate structures before reaching the final state. However, our knowledge on the exact pathways of this process is limited, mainly owing to the difficulty of realizing direct observations. Here, we experimentally study the evolution of symmetry and density for various colloidal systems during liquid-to-solid phase transitions, and visualize kinetic pathways with single-particle resolution. We observe the formation of relatively ordered precursor structures with different symmetries, which then convert into metastable solids. During this conversion, two major cross-symmetry pathways always occur, regardless of the final state and the interaction potential. In addition, we find a broad decoupling of density variation and symmetry development, and discover that nucleation rarely starts from the densest regions. These findings hold for all of our samples, suggesting the possibility of finding a unified picture for the complex crystallization kinetics in colloidal systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ostwald, W. Studien über die Bildung und Umwandlung fester Körper. 1. Abhandlung: Übersättigung und Überkaltung. Z. Phys. Chem. 22, 289–330 (1897).
Alexander, S. & McTague, J. Should all crystals be bcc? Landau theory of solidification and crystal nucleation. Phys. Rev. Lett. 41, 702–705 (1978).
Ten Wolde, P. R., Ruiz-Montero, M. J. & Frenkel, D. Numerical evidence for bcc ordering at the surface of a critical fcc nucleus. Phys. Rev. Lett. 75, 2714–2717 (1995).
Ten Wolde, P. R., Ruiz-Montero, M. J. & Frenkel, D. Numerical calculation of the rate of crystal nucleation in a Lennard-Jones system at moderate undercooling. J. Chem. Phys. 104, 9932–9947 (1996).
Shen, Y. C. & Oxtoby, D. W. bcc symmetry in the crystal-melt interface of Lennard-Jones fluids examined through density functional theory. Phys. Rev. Lett. 77, 3585–3588 (1996).
Auer, S. & Frenkel, D. Crystallization of weakly charged colloidal spheres: A numerical study. J. Phys. Condens. Matter 14, 7667–7680 (2002).
Moroni, D., ten Wolde, P. R. & Bolhuis, P. G. Interplay between structure and size in a critical crystal nucleus. Phys. Rev. Lett. 94, 235703 (2005).
Russo, J. & Tanaka, H. Selection mechanism of polymorphs in the crystal nucleation of the Gaussian core model. Soft Matter 8, 4206–4215 (2012).
Pusey, P. N. & van Megen, W. Phase behaviour of concentrated suspensions of nearly hard colloidal spheres. Nature 320, 340–342 (1986).
Pusey, P. N. et al. Structure of crystals of hard colloidal spheres. Phys. Rev. Lett. 63, 2753–2756 (1989).
Zhu, J. et al. Crystallization of hard-sphere colloids in microgravity. Nature 387, 883–885 (1997).
Gasser, U., Weeks, E. R., Schofield, A., Pusey, P. N. & Weitz, D. A. Real-space imaging of nucleation and growth in colloidal crystallization. Science 292, 258–262 (2001).
Auer, S. & Frenkel, D. Prediction of absolute crystal-nucleation rate in hard-sphere colloids. Nature 409, 1020–1023 (2001).
TenWolde, P. R. & Frenkel, D. Enhancement of protein crystal nucleation by critical density fluctuations. Science 277, 1975–1978 (1997).
Kawasaki, T. & Tanaka, H. Formation of a crystal nucleus from liquid. Proc. Natl Acad. Sci. USA 107, 14036–14041 (2010).
Russo, J. & Tanaka, H. The microscopic pathway to crystallization in supercooled liquids. Sci. Rep. 2, 505 (2012).
Schilling, T., Schöpe, H. J., Oettel, M., Opletal, G. & Snook, I. Precursor-mediated crystallization process in suspensions of hard spheres. Phys. Rev. Lett. 105, 025701 (2010).
Tóth, G. I., Pusztai, T., Tegze, G., Tóth, G. & Gránásy, L. Amorphous nucleation precursor in highly nonequilibrium fluids. Phys. Rev. Lett. 107, 175702 (2011).
Lechner, W., Dellago, C. & Bolhuis, P. G. Role of the prestructured surface cloud in crystal nucleation. Phys. Rev. Lett. 106, 085701 (2011).
Lutsko, J. F. & Nicolis, G. Theoretical evidence for a dense fluid precursor to crystallization. Phys. Rev. Lett. 96, 046102 (2006).
Martin, S., Bryant, G. & van Megen, W. Crystallization kinetics of polydisperse colloidal hard spheres: Experimental evidence for local fractionation. Phys. Rev. E 67, 061405 (2003).
Schöpe, H. J., Bryant, G. & van Megen, W. Two-step crystallization kinetics in colloidal hard-sphere systems. Phys. Rev. Lett. 96, 175701 (2006).
Iacopini, S., Palberg, T. & Schöpe, H. J. Crystallization kinetics of polydisperse hard-sphere-like microgel colloids: Ripening dominated crystal growth above melting. J. Chem. Phys. 130, 084502 (2009).
Savage, J. R. & Dinsmore, A. D. Experimental evidence for two-step nucleation in colloidal crystallization. Phys. Rev. Lett. 102, 198302 (2009).
Larsen, A. E. & Grier, D. G. Like-charge attractions in metastable colloidal crystallites. Nature 385, 230–233 (1997).
Weeks, E. R., Crocker, J. C., Levitt, A. C., Schofield, A. & Weitz, D. A. Three-dimensional direct imaging of structural relaxation near the colloidal glass transition. Science 287, 627–631 (2000).
Anderson, V. J. & Lekkerkerker, H. N. Insights into phase transition kinetics from colloid science. Nature 416, 811–815 (2002).
Schall, P., Cohen, I., Weitz, D. A. & Spaepen, F. Visualization of dislocation dynamics in colloidal crystals. Science 305, 1944–1948 (2004).
Alsayed, A. M., Islam, M. F., Zhang, J., Collings, P. J. & Yodh, A. G. Premelting at defects within bulk colloidal crystals. Science 309, 1207–1210 (2005).
Savage, J. R., Blair, D. W., Levine, A. J., Guyer, R. A. & Dinsmore, A. D. Imaging the sublimation dynamics of colloidal crystallites. Science 314, 795–798 (2006).
Lu, P. J., Zaccarelli, E., Ciulla, F., Schofield, A. B., Sciortino, F. & Weitz, D. A. Gelation of particles with short-range attraction. Nature 453, 499–503 (2008).
Wang, Z. R., Wang, F., Peng, Y., Zheng, Z. Y. & Han, Y. L. Imaging the homogeneous nucleation during the melting of superheated colloidal crystals. Science 338, 87–90 (2012).
Leunissen, M. E. et al. Ionic colloidal crystals of oppositely charged particles. Nature 437, 235–240 (2005).
Tan, P., Xu, N., Schofield, A. & Xu, L. Understanding the low-frequency quasilocalized modes in disordered colloidal systems. Phys. Rev. Lett. 108, 095501 (2012).
Hynninen, A. & Dijkstra, M. Phase diagrams of hard-core repulsive Yukawa particles. Phys. Rev. E 68, 021407 (2003).
Steinhardt, P. J., Nelson, D. R. & Ronchetti, M. Bond-orientational order in liquids and glasses. Phys. Rev. B 28, 784–805 (1983).
Ten Wolde, P., Ruiz-Montero, M. J. & Frenkel, D. Simulation of homogeneous crystal nucleation close to coexistence. Faraday Discuss. 104, 93–110 (1996).
Lechner, W. & Dellago, C. Crystal structures based on averaged local bond order parameters. J. Chem. Phys. 129, 114707 (2008).
Anikeenko, A. V. & Medvedev, N. N. Polytetrahedral nature of the dense disordered packings of hard spheres. Phys. Rev. Lett. 98, 235504 (2007).
Meijer, E. J. & Frenkel, D. Melting line of Yukawa system by computer simulation. J. Chem. Phys. 94, 2269–2271 (1991).
Zahn, K. & Maret, G. Dynamic criteria for melting in two dimensions. Phys. Rev. Lett. 85, 3656–3659 (2000).
Acknowledgements
P.T. and L.X. are supported by the Research Grants Council of Hong Kong (GRF grant CUHK404211, ECS grant CUHK404912, CUHK Direct Grant 4053021), and N.X. is supported by the National Natural Science Foundation of China (No. 91027001 and 11074228), the National Basic Research Program of China (973 Program No. 2012CB821500), the CAS 100-Talent Program (No. 2030020004), and Fundamental Research Funds for the Central Universities (No. 2340000034). We thank H. Tanaka and E. Sloutskin for helpful discussions, and A. Schofield for providing the particles.
Author information
Authors and Affiliations
Contributions
P.T. and L.X. conceived and designed the experiments, P.T. performed the experiments, P.T., N.X. and L.X. analysed the data, P.T. developed the new approach of local bond order analysis, and P.T. and L.X. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1186 kb)
Supplementary Movie
Supplementary Movie 1 (AVI 4169 kb)
Supplementary Movie
Supplementary Movie 2 (AVI 4101 kb)
Supplementary Movie
Supplementary Movie 3 (AVI 4423 kb)
Rights and permissions
About this article
Cite this article
Tan, P., Xu, N. & Xu, L. Visualizing kinetic pathways of homogeneous nucleation in colloidal crystallization. Nature Phys 10, 73–79 (2014). https://doi.org/10.1038/nphys2817
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphys2817
This article is cited by
-
Non-classical crystallization in soft and organic materials
Nature Reviews Materials (2024)
-
Lithium crystallization at solid interfaces
Nature Communications (2023)
-
Microscopic ordering of supercooled water on the ice basal face
Communications Materials (2023)
-
Ab initio Molecular Dynamics Study of Local Atomic Structure Evolution of U–Zr Alloy Melts upon Solidification
Acta Metallurgica Sinica (English Letters) (2023)
-
Revealing the role of liquid preordering in crystallisation of supercooled liquids
Nature Communications (2022)