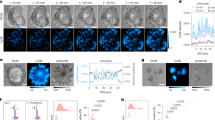
Abstract
We introduce an optical platform for rapid, high-throughput screening of exogenous molecules that affect cellular mechanotransduction. Our method initiates mechanotransduction in adherent cells using single laser-microbeam generated microcavitation bubbles without requiring flow chambers or microfluidics. These microcavitation bubbles expose adherent cells to a microtsunami, a transient microscale burst of hydrodynamic shear stress, which stimulates cells over areas approaching 1 mm2. We demonstrate microtsunami-initiated mechanosignalling in primary human endothelial cells. This observed signalling is consistent with G-protein-coupled receptor stimulation, resulting in Ca2+ release by the endoplasmic reticulum. Moreover, we demonstrate the dose-dependent modulation of microtsunami-induced Ca2+ signalling by introducing a known inhibitor to this pathway. The imaging of Ca2+ signalling and its modulation by exogenous molecules demonstrates the capacity to initiate and assess cellular mechanosignalling in real time. We utilize this capability to screen the effects of a set of small molecules on cellular mechanotransduction in 96-well plates using standard imaging cytometry.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wang, Y. et al. Visualizing the mechanical activation of Src. Nature 434, 1040–1045 (2005).
Peyton, S. R., Raub, C. B., Keschrumrus, V. P. & Putnam, A. J. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials 27, 4881–4893 (2006).
Kumar, S. et al. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys. J. 90, 3762–3773 (2006).
Discher, D. E., Janmey, P. & Wang, Y. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005).
Ingber, D. E. Tensegrity: the architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 59, 575–599 (1997).
Chien, S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am. J. Physiol. Heart Circ. Physiol. 292, H1209–H1224 (2007).
Jaalouk, D. E. & Lammerding, J. Mechanotransduction gone awry. Nature Rev. Mol. Cell Biol. 10, 63–73 (2009).
Orr, A. W., Helmke, B. P., Blackman, B. R. & Schwartz, M. A. Mechanisms of mechanotransduction. Dev. Cell 10, 11–20 (2006).
Ingber, D. E. Mechanobiology and diseases of mechanotransduction. Ann. Med. 35, 564–577 (2003).
Hahn, C. & Schwartz, M. A. Mechanotransduction in vascular physiology and atherogenesis. Nature Rev. Mol. Cell Biol. 10, 53–62 (2009).
Gottlieb, P. A., Suchyna, T. M., Ostrow, L. W. & Sachs, F. Mechanosensitive ion channels as drug targets. Curr. Drug Targets CNS Neurol. Disord. 3, 287–295 (2004).
Huh, D., Hamilton, G. A. & Ingber, D. E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 21, 745–754 (2011).
Apic, G., Ignjatovic, T., Boyer, S. & Russell, R. B. Illuminating drug discovery with biological pathways. FEBS Lett. 579, 1872–1877 (2005).
Drews, J. Drug discovery: a historical perspective. Science 287, 1960–1964 (2000).
Rudin, M. & Weissleder, R. Molecular imaging in drug discovery and development. Nature Rev. Drug Discov. 2, 123–131 (2003).
Charras, G. T. & Horton, M. A. Single cell mechanotransduction and its modulation analyzed by atomic force microscope indentation. Biophys. J. 82, 2970–2981 (2002).
Chien, S. Effects of disturbed flow on endothelial cells. Ann. Biomed. Eng. 36, 554–562 (2008).
Valberg, P. A. & Butler, J. P. Magnetic particle motions within living cells. Physical theory and techniques. Biophys. J. 52, 537–550 (1987).
Chen, C. S., Mrksich, M., Huang, S., Whitesides, G. M. & Ingber, D. E. Geometric control of cell life and death. Science 276, 1425–1428 (1997).
Shergill, B., Meloty-Kapella, L., Musse, A. A., Weinmaster, G. & Botvinick, E. Optical tweezers studies on notch: single-molecule interaction strength is independent of ligand endocytosis. Dev. Cell 22, 1313–1320 (2012).
Meloty-Kapella, L., Shergill, B., Kuon, J., Botvinick, E. & Weinmaster, G. Notch ligand endocytosis generates mechanical pulling force dependent on dynamin, epsins, and actin. Dev. Cell 22, 1299–1312 (2012).
Rau, K. R., Quinto-Su, P. A., Hellman, A. N. & Venugopalan, V. Pulsed laser microbeam-induced cell lysis: time-resolved imaging and analysis of hydrodynamic effects. Biophys. J. 91, 317–329 (2006).
Hellman, A. N., Rau, K. R., Yoon, H. H. & Venugopalan, V. Biophysical response to pulsed laser microbeam-induced cell lysis and molecular delivery. J. Biophoton. 1, 24–35 (2008).
Davies, P. F. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 75, 519–560 (1995).
Tran, Q.-K. & Watanabe, H. Calcium signalling in the endothelium. Handb. Exp. Pharmacol. 2, 145–187 (2006).
Kuchan, M. J. & Frangos, J. A. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am. J. Physiol. 266, C628–C636 (1994).
Palmer, A. E. & Tsien, R. Y. Measuring calcium signaling using genetically targetable fluorescent indicators. Nature Protoc. 1, 1057–1065 (2006).
Liu, B., Lu, S., Zheng, S., Jiang, Z. & Wang, Y. Two distinct phases of calcium signalling under flow. Cardiovasc. Res. 91, 124–133 (2011).
Vogel, A. & Venugopalan, V. Mechanisms of pulsed laser ablation of biological tissues. Chem. Rev. 103, 577–644 (2003).
Vogel, A., Noack, J., Hüttman, G. & Paltauf, G. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl. Phys. B 81, 1015–1047 (2005).
Venugopalan, V., Guerra, A., Nahen, K. & Vogel, A. Role of laser-induced plasma formation in pulsed cellular microsurgery and micromanipulation. Phys. Rev. Lett. 88, 078103 (2002).
Vogel, A. Nonlinear absorption: intraocular microsurgery and laser lithotripsy. Phys. Med. Biol. 42, 895–912 (1997).
Stevenson, D. J., Gunn-Moore, F. J., Campbell, P. & Dholakia, K. Single cell optical transfection. J. R. Soc. Interface 7, 863–871 (2010).
Gilmore, F. R. The Growth or Collapse of a Spherical Bubble in a Viscous Liquid (Office of Naval Research, 1952).
Knapp, R. T., Daily, J. W. & Hammitt, F. G. Cavitation (McGraw-Hill, 1970).
Vogel, A., Busch, S. & Parlitz, U. Shock wave emission and cavitation bubble generation by picosecond and nanosecond optical breakdown in water. J. Acoust. Soc. Am. 100, 148 (1996).
Vogel, A., Linz, N., Freidank, S. & Paltauf, G. Femtosecond-laser-induced nanocavitation in water: implications for optical breakdown threshold and cell surgery. Phys. Rev. Lett. 100, 038102 (2008).
Compton, J. L., Hellman, A. N. & Venugopalan, V. Hydrodynamic determinants of cell necrosis and molecular delivery produced by pulsed laser microbeam irradiation of adherent cells. Biophys. J. 105, 2221–2231 (2013).
Malek, A. M., Alper, S. L. & Izumo, S. Hemodynamic shear stress and its role in atherosclerosis. Am. J. Physiol. 282, 2035–2042 (1999).
Bishara, N. B., Murphy, T. V. & Hill, M. A. Capacitative Ca2+ entry in vascular endothelial cells is mediated via pathways sensitive to 2-aminoethoxydiphenyl borate and xestospongin C. Br. J. Pharmacol. 135, 119–128 (2002).
Demer, L. L., Wortham, C. M., Dirksen, E. R. & Sanderson, M. J. Mechanical stimulation induces intercellular calcium signaling in bovine aortic endothelial cells. Am. J. Physiol. 264, H2094–H2102 (1993).
Seong, J., Lu, S. & Wang, Y. Live cell imaging of Src/FAK signaling by FRET. Cell. Mol. Bioeng. 4, 138–147 (2011).
Cherian, A. V. & Rau, K. R. Pulsed-laser-induced damage in rat corneas: time-resolved imaging of physical effects and acute biological response. J. Biomed. Opt. 13, 024009 (2008).
Edelstein, A. et al., Amodaj, N., Hoover, K., Vale, R. & Stuurman, N. in Current Protocols in Molecular Biology (eds Ausubel, F. M. et al.) Ch. 14, Unit 14.20 (Wiley, 2010).
Palmer, A. E. & Tsien, R. Y. Measuring calcium signaling using genetically targetable fluorescent indicators. Nature Protoc. 1, 1057–1065 (2006).
Park, J. W., Vahidi, B., Taylor, A. M., Rhee, S. W. & Jeon, N. L. Microfluidic culture platform for neuroscience research. Nature Protoc. 1, 2128–2136 (2006).
Yang, M. T., Fu, J., Wang, Y.-K., Desai, R. A. & Chen, C. S. Assaying stem cell mechanobiology on microfabricated elastomeric substrates with geometrically modulated rigidity. Nature Protoc. 6, 187–213 (2011).
Acknowledgements
The authors thank A. Hellman for her early contributions to this research, M. Khine for assistance with microfabrication and C. Hughes for supplying HUVECs. This research was funded through the Laser Microbeam and Medical Program, a National Biomedical Technology Resource (P41-EB015890) supported by the National Institutes of Health, the National Science Foundation through the Integrative Graduate Education and Research Traineeship (IGERT) Program (DGE-1144901), and UC Irvine through the I3 Award Program.
Author information
Authors and Affiliations
Contributions
E.B. and V.V. conceived the project. J.L.C., J.C.L., E.B. and V.V. designed the experiments. J.L.C., J.C.L. and H.M. performed the experiments. J.L.C. and V.V. developed the hydrodynamic model. J.L.C., J.C.L., E.B. and V.V. performed the data analysis. J.L.C., J.C.L., E.B. and V.V. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Authors Compton, Botvinick and Venugopalan filed a non-provisional US patent application “Mechanical Stress Response Analysis of Cells and Tissues” Serial No. 14/046,804 on 4 October 2013 based on the findings of this study.
Supplementary information
Supplementary information
Supplementary information (PDF 800 kb)
Rights and permissions
About this article
Cite this article
Compton, J., Luo, J., Ma, H. et al. High-throughput optical screening of cellular mechanotransduction. Nature Photon 8, 710–715 (2014). https://doi.org/10.1038/nphoton.2014.165
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphoton.2014.165
This article is cited by
-
Laser cavitation rheology for measurement of elastic moduli and failure strain within hydrogels
Scientific Reports (2020)
-
Plenty more room on the glass bottom: Surface functionalization and nanobiotechnology for cell isolation
Nano Research (2018)
-
Mechanical forces direct stem cell behaviour in development and regeneration
Nature Reviews Molecular Cell Biology (2017)
-
Near-unity quantum efficiency of broadband black silicon photodiodes with an induced junction
Nature Photonics (2016)
-
Monitoring the intracellular calcium response to a dynamic hypertonic environment
Scientific Reports (2016)