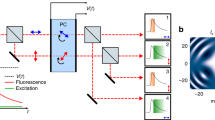
Abstract
Fluorescence imaging is the most widely used method for unveiling the molecular composition of biological specimens. However, the weak optical emission of fluorescent probes and the trade-off between imaging speed and sensitivity1 are problematic for acquiring blur-free images of fast phenomena, such as sub-millisecond biochemical dynamics in live cells and tissues2, and cells flowing at high speed3. Here, we report a technique that achieves real-time pixel readout rates that are one order of magnitude faster than a modern electron multiplier charge-coupled device—the gold standard in high-speed fluorescence imaging technology4. Termed fluorescence imaging using radiofrequency-tagged emission (FIRE), this approach maps the image into the radiofrequency spectrum using the beating of digitally synthesized optical fields. We demonstrate diffraction-limited confocal fluorescence imaging of stationary cells at a frame rate of 4.4 kHz, and fluorescence microscopy in flow at a velocity of 1 m s−1, corresponding to a throughput of approximately 50,000 cells per second.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lakowicz, J. R. Principles of Fluorescence Spectroscopy 3rd edn (Springer, 2006).
Scanziani, M. & Hausser, M. Electrophysiology in the age of light. Nature 461, 930–939 (2009).
Elliott, G. S. Moving pictures: imaging flow cytometry for drug development. Comb. Chem. High. T. Scr. 12, 849–859 (2009).
Coates, C. New sCMOS vs. Current Microscopy Cameras, Andor white paper (Andor, 2011).
Huang, B., Bates, M. & Zhuang, X. W. Super-resolution fluorescence microscopy. Annu. Rev. Biochem. 78, 993–1016 (2009).
Tsien, R. Y. Fluorescent indicators of ion concentrations. Methods Cell Biol. 30, 127–156 (1989).
Petty, H. R. Spatiotemporal chemical dynamics in living cells: from information trafficking to cell physiology. Biosystems 83, 217–224 (2006).
Grinvald, A., Anglister, L., Freeman, J. A., Hildesheim, R. & Manker, A. Real-time optical imaging of naturally evoked electrical-activity in intact frog brain. Nature 308, 848–850 (1984).
Cheng, A., Goncalves, J. T., Golshani, P., Arisaka, K. & Portera-Cailliau, C. Simultaneous two-photon calcium imaging at different depths with spatiotemporal multiplexing. Nature Methods 8, 139–158 (2011).
Yuste, R. & Denk, W. Dendritic spines as basic functional units of neuronal integration. Nature 375, 682–684 (1995).
Shiferaw, Y., Aistrup, G. L. & Wasserstrom, J. A. Intracellular Ca2+ waves, afterdepolarizations, and triggered arrhythmias. Cardiovasc. Res. 95, 265–268 (2012).
George, T. C. et al. Quantitative measurement of nuclear translocation events using similarity analysis of multispectral cellular images obtained in flow. J. Immunol. Methods 311, 117–129 (2006).
Khalil, A. M., Cambier, J. C. & Shlomchik, M. J. B cell receptor signal transduction in the GC is short-circuited by high phosphatase activity. Science 336, 1178–1181 (2012).
Pawley, J. B. Handbook of Biological Confocal Microscopy (Springer, 2006).
Goda, K., Tsia, K. K. & Jalali, B. Serial time-encoded amplified imaging for real-time observation of fast dynamic phenomena. Nature 458, 1145–1149 (2009).
Goda, K. et al. High-throughput single-microparticle imaging flow analyzer. Proc. Natl Acad. Sci. USA 109, 11630–11635 (2012).
Basiji, D. A. & Ortyn, W. E. Imaging and analyzing parameters of small moving objects such as cells. US patent 6,211,955 (2001).
Allard, W. J. et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 10, 6897–6904 (2004).
Nagrath, S. et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450, 1235–1239 (2007).
Howard, S. S., Straub, A., Horton, N. G., Kobat, D. & Xu, C. Frequency-multiplexed in vivo multiphoton phosphorescence lifetime microscopy. Nature Photon. 7, 33–37 (2013).
Wu, F. et al. Frequency division multiplexed multichannel high-speed fluorescence confocal microscope. Biophys. J. 91, 2290–2296 (2006).
Sanders, J. S., Driggers, R. G., Halford, C. E. & Griffin, S. T. Imaging with frequency-modulated reticles. Opt. Eng. 30, 1720–1724 (1991).
Futia, G., Schlup, P., Winters, D. G. & Bartels, R. A. Spatially-chirped modulation imaging of absorbtion and fluorescent objects on single-element optical detector. Opt. Express 19, 1626–1640 (2011).
Young, E. H. & Yao, S. K. Design considerations for acoustooptic devices. Proc. IEEE 69, 54–64 (1981).
Nee, R. v. & Prasad, R. OFDM for Wireless Multimedia Communications (Artech House, 2000).
Kim, K. H. et al. Multifocal multiphoton microscopy based on multianode photomultiplier tubes. Opt. Express 15, 11658–11678 (2007).
Kralj, J. M., Douglass, A. D., Hochbaum, D. R., Maclaurin, D. & Cohen, A. E. Optical recording of action potentials in mammalian neurons using a microbial rhodopsin. Nature Methods 9, 90–95 (2012).
Golay, M. J. E. Complementary series. Ire. T. Inform. Theor. 7, 82 (1961).
Lakowicz, J. R. & Berndt, K. W. Lifetime-selective fluorescence imaging using an RF phase-sensitive camera. Rev. Sci. Instrum. 62, 1727–1734 (1991).
Acknowledgements
The authors thank D. Di Carlo (UCLA) for use of his laboratory's cell culture facilities. The authors acknowledge the Broad Stem Cell Research Center at UCLA 2012 Innovation Award for financial support. The authors also thank L. Bentolila for assistance with the EMCCD imaging, which was performed at the California NanoSystems Institute Advanced Light Microscopy/Spectroscopy Shared Facility at UCLA.
Author information
Authors and Affiliations
Contributions
E.D.D. conceived of the beat frequency multiplexing approach, built the FIRE microscope, and collected the data. B.W.B. conceived of and implemented the demodulation algorithms, generated the phase engineered excitation frequency combs, and performed image processing. D.R.G. cultured and stained the biological samples, and fabricated microfluidic channels. B.J. conceived of the use of DDS and other communication techniques for FIRE, and supervised the project. E.D.D. wrote the first draft of the manuscript, and all authors contributed to subsequent revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1085 kb)
Supplementary Movie
Supplementary Movie (MOV 3158 kb)
Rights and permissions
About this article
Cite this article
Diebold, E., Buckley, B., Gossett, D. et al. Digitally synthesized beat frequency multiplexing for sub-millisecond fluorescence microscopy. Nature Photon 7, 806–810 (2013). https://doi.org/10.1038/nphoton.2013.245
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphoton.2013.245
This article is cited by
-
Imaging flow cytometry
Nature Reviews Methods Primers (2022)
-
Acousto-optically driven lensless single-shot ultrafast optical imaging
Light: Science & Applications (2022)
-
Light sheet based volume flow cytometry (VFC) for rapid volume reconstruction and parameter estimation on the go
Scientific Reports (2022)
-
Dual sequentially addressable dielectrophoretic array for high-throughput, scalable, multiplexed droplet sorting
Microfluidics and Nanofluidics (2021)
-
Virtual-freezing fluorescence imaging flow cytometry
Nature Communications (2020)