Abstract
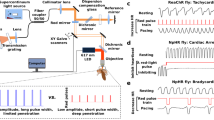
Light has been used to non-invasively alter the excitability of both neural and cardiac tissue1,2,3,4,5,6,7,8,9,10. Recently, pulsed laser light has been shown to be capable of eliciting action potentials in peripheral nerves and in cultured cardiomyocytes7,8,9,10. Here, for the first time, we demonstrate optical pacing of an intact heart in vivo. Pulsed 1.875-µm infrared laser light was used to lock the heart rate to the pulse frequency of the laser. A laser Doppler velocimetry signal was used to verify the pacing. At low radiant exposures, embryonic quail hearts were reliably paced in vivo without detectable damage to the tissue, indicating that optical pacing has great potential as a tool with which to study embryonic cardiac dynamics and development. In particular, optical pacing can be used to control the heart rate, thereby altering stresses and mechanically transduced signalling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Allegre, G., Avrillier, S. & Albe-Fessard, D. Stimulation in the rat of a nerve fiber bundle by a short UV pulse from an excimer laser. Neurosci. Lett. 180, 261–264 (1994).
Balaban, P. et al. He–Ne laser irradiation of single identified neurons. Lasers Surg. Med. 12, 329–337, (1992).
Fork, R. L. Laser stimulation of nerve cells in aplysia. Science 171, 907–908 (1971).
Gimeno, M. A., Robets, C. M. & Webb, J. L. Acceleration of rate of the early chick embryo heart by visible light. Nature 214, 1014–1016 (1967).
Hirase, H., Nikolenko, V., Goldberg, J. H. & Yuste, R. Multiphoton stimulation of neurons. J. Neurobiol. 51, 237–247 (2002).
Nathan, R. D., Pooler, J. P. & DeHaan, R. L. Ultraviolet-induced alterations of beat rate and electrical properties of embryonic chick heart cell aggregates. J. Gen. Physiol. 67, 27–44 (1976).
Smith, N. I. et al. A femtosecond laser pacemaker for heart muscle cells. Opt. Express 16, 8604–8616 (2008).
Wells, J., Kao, C., Jansen, E. D., Konrad, P. & Mahadevan-Jansen, A. Application of infrared light for in vivo neural stimulation. J. Biomed. Opt. 10, 064003 (2005).
Wells, J. et al. Biophysical mechanisms of transient optical stimulation of peripheral nerve. Biophys. J. 93, 2567–2580 (2007).
Wells, J. et al. Optical stimulation of neural tissue in vivo. Opt. Lett. 30, 504–506 (2005).
Bartman, T. & Hove, J. Mechanics and function in heart morphogenesis. Dev. Dyn. 233, 373–381 (2005).
North, T. E. et al. Hematopoietic stem cell development is dependent on blood flow. Cell 137, 736–748 (2009).
Pardanaud, L. & Eichmann, A. Stem cells: the stress of forming blood cells. Nature 459, 1068–1069 (2009).
Poelmann, R. E., Gittenberger-de Groot, A. C. & Hierck, B. P. The development of the heart and microcirculation: role of shear stress. Med. Biol. Eng. Comput. 46, 479–484 (2008).
Wells, J. D. et al. Optically mediated nerve stimulation: identification of injury thresholds. Lasers Surg. Med. 39, 513–526, (2007).
New, D. A. T. A new technique for the cultivation of the chick embryo in vitro. J. Embryol. Exp. Morphol. 3, 326–331 (1955).
Gargesha, M., Jenkins, M. W., Wilson, D. L. & Rollins, A. M. High temporal resolution OCT using image-based retrospective gating. Opt. Express 17, 10786–10799 (2009).
Jenkins, M. W. et al. in BiOS (SPIE, 2008).
Jenkins, M. W. et al. Ultrahigh-speed optical coherence tomography imaging and visualization of the embryonic avian heart using a buffered Fourier domain mode Locked laser. Opt. Express 15, 6251–6267 (2007).
Jenkins, M. W. et al. in BiOS (SPIE, 2009).
Darnell, D. K. & Schoenwolf, G. C. in Methods in Molecular Biology Vol. 135 (eds Tuan, R. S. & Lo, C. W.) Ch. 5, 31–38 (Humana Press, 2000).
Kalt, M. R. & Tandler, B. A study of fixation of early amphibian embryos for electron microscopy. J. Ultrastruct. Res. 36, 633–645 (1971).
Karnovsky, M. J. Use of ferrocyanide-reduced osmium tetroxide in electron microscopy. Abstracts of Papers, Eleventh Annual Meeting, New Orleans, LA, 146 (American Society for Cell Biology, 1971)
Tandler, B. Improved uranyl acetate staining for electron microscopy. J. Electron Microsc. Tech. 16, 81–82 (1990).
Hanaichi, T. et al. A stable lead by modification of Sato's method. J. Electron Microsc. 35, 304–306 (1986).
Acknowledgements
This research was supported in part by the National Institutes of Health (RO1-HL083048 (A.M.R.), RO1-HL095717 (A.M.R.), RO1-NS052407 (E.D.J.) and R44-NS051926 (E.D.J.). This investigation was conducted in a facility constructed with support from the Research Facilities Improvement Program grant no. C06 RR12463-01 from the National Center of Research Resources, National Institutes of Health. The authors appreciate the contributions of M. Hitomi in preparing embryos for TEM.
Author information
Authors and Affiliations
Contributions
M.W.J. conceived the original idea, performed and designed the experiments, analysed data and wrote the paper. A.R.D and S.G. performed and designed experiments and analysed data. Y.D. prepared and performed histology on the embryos and helped in preparing the embryos for TEM. H.J.C designed experiments and analysed data. H.F. supervised analysis and the creation of micrographs. M.W. supervised damage studies and embryo handling. E.D.J. and A.M.R. supervised optical pacing experiments. All authors helped to edit the paper. All authors except H.F. and Y.D. discussed the results and implications at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information Figure (PDF 508 kb)
Supplementary information
Supplementary information Movie (MOV 9586 kb)
Rights and permissions
About this article
Cite this article
Jenkins, M., Duke, A., Gu, S. et al. Optical pacing of the embryonic heart. Nature Photon 4, 623–626 (2010). https://doi.org/10.1038/nphoton.2010.166
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphoton.2010.166
This article is cited by
-
Skeletal muscle cells opto-stimulation by intramembrane molecular transducers
Communications Biology (2023)
-
Neural modulation with photothermally active nanomaterials
Nature Reviews Bioengineering (2023)
-
Infrared neural stimulation markedly enhances nerve functionality assessment during nerve monitoring
Scientific Reports (2023)
-
Porosity-based heterojunctions enable leadless optoelectronic modulation of tissues
Nature Materials (2022)
-
Bidirectional modulation of evoked synaptic transmission by pulsed infrared light
Scientific Reports (2022)