Abstract
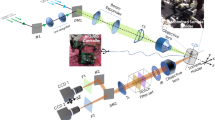
Fluorescent proteins have become essential reporter molecules for studying life at the cellular and sub-cellular level, re-defining the ways in which we investigate biology. However, because of intense light scattering, most organisms and tissues remain inaccessible to current fluorescence microscopy techniques at depths beyond several hundred micrometres. We describe a multispectral opto-acoustic tomography technique capable of high-resolution visualization of fluorescent proteins deep within highly light-scattering living organisms. The method uses multiwavelength illumination over multiple projections combined with selective-plane opto-acoustic detection for artifact-free data collection. Accurate image reconstruction is enabled by making use of wavelength-dependent light propagation models in tissue. By performing whole-body imaging of two biologically important and optically diffuse model organisms, Drosophila melanogaster pupae and adult zebrafish, we demonstrate the facility to resolve tissue-specific expression of eGFP and mCherrry fluorescent proteins for precise morphological and functional observations in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Giepmans, B. N. G., Adams, S. R., Ellisman, M. H. & Tsien, R. Y. The fluorescent toolbox for assessing protein location and function. Science 312, 217–224 (2006).
Lichtman, J. W. & Conchello, J. A. Fluorescence microscopy. Nature Methods 2, 910–919 (2005).
Conchello J. A. & Lichtman, J. W. Optical sectioning microscopy. Nature Methods 2, 920–931 (2005).
Bahlmann, K. et al. Multifocal multiphoton microscopy (MMM) at a frame rate beyond 600 Hz. Opt. Express 15, 10991–10998 (2007).
Minsky, M. Microscopy apparatus. US patent 3,013,467 (1961).
Denk, W., Strickler, J. H. & Webb, W. W. 2-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Helmchen, F. & Denk, W. Deep tissue two-photon microscopy. Nature Methods 2, 932–940 (2005).
Ntziachristos, V., Ripoll, J., Wang, L. H. V. & Weissleder, R. Looking and listening to light: the evolution of whole-body photonic imaging. Nature Biotechnol. 23, 313–320 (2005).
Hove, J. R. et al. Intracardiac hemodynamics are an essential epigenetic factor for embryonic cardiogenesis. Nature 421, 172–177 (2003).
Jain, R. K., Munn, L. L. & Fukumura, D. Dissecting tumor pathophysiology using intravital microscopy. Nature Rev. Cancer 2, 266–276 (2002).
Sharpe, J. et al. Optical projection tomography as a tool for 3D microscopy and gene expression studies. Science 296, 541–545 (2002).
Huisken, J. et al. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 305, 1007–1009 (2004).
Dodt, H. U. et al. Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nature Methods 4, 331–336 (2007).
Editorial. Geneticist seeks engineer: must like flies and worms. Nature Methods 4, 463 (2007).
Schroeder, T. Imaging stem-cell-driven regeneration in mammals. Nature 453, 345–351 (2008).
Gusev, V. E. & Karabutov, A. A. Laser Optoacoustics (American Institute of Physics, 1993).
Zhang, E. Z., Laufer, J. G., Pedley, R. B. & Beard, P. C. In vivo high-resolution 3D photoacoustic imaging of superficial vascular anatomy. Phys. Med. Biol. 54, 1035–1046 (2009).
Lao, Y., Xing, D., Yang, S. & Xiang, L. Noninvasive photoacoustic imaging of the developing vasculature during early tumor growth. Phys. Med. Biol. 53, 4203–4212 (2008).
Wang, X. et al. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nature Biotechnol. 21, 803–806 (2003).
Zhang, H. F., Maslov, K., Stoica, G. & Wang, L. V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nature Biotechnol. 24, 848–851 (2006).
Razansky, D., Vinegoni, C. & Ntziachristos, V. Multispectral photoacoustic imaging of fluorochromes in small animals. Opt. Lett. 32, 2891–2893 (2007).
Li, L., Zemp, R. J., Lungu, G., Stoica, G. & Wang, L. V. Photoacoustic imaging of lacZ gene expression in vivo. J. Biomed. Opt. 12, 020504 (2007).
De La Zerda, A. et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nature Nanotechnol. 3, 557–562 (2008).
Razansky, D., Baeten, J. & Ntziachristos, V. Sensitivity of molecular target detection by multispectral optoacoustic tomography (MSOT). Med. Phys. 36, 2891–2893 (2009).
Vinegoni, C., Pitsouli, C., Razansky, D., Perrimon, N. & Ntziachristos, V. In vivo imaging of Drospophila melanogaster pupae with mesoscopic fluorescence tomography. Nature Methods 5, 45–47 (2008).
Ntziachristos, V., Tung, C.-H., Bremer, C. & Weissleder, R. Fluorescence molecular tomography resolves protease activity in vivo. Nature Med. 8, 757–760 (2002).
Xu, M. & Wang, L. V. Universal back-projection algorithm for photoacoustic computed tomography. Phys. Rev. E 71, 016706 (2005).
Razansky, D. & Ntziachristos, V. Hybrid photoacoustic fluorescence molecular tomography using finite-element-based inversion. Med. Phys. 34, 4293–4301 (2007).
Cox, B. T., Arridge, S. R., Kostli, K. P. & Beard, P. C. 2D quantitative photoacoustic image reconstruction of absorption distributions in scattering media using a simple iterative method. Appl. Opt. 45, 1866–1875 (2006).
Jetzfellner, T. et al. Iterative optoacoustic image normalization in non-uniform illumination configurations. Appl. Phys. Lett. 95, (2009) (in the press).
Laufer, J. G., Delpy, D. T., Elwell, C. E. & Beard, P. C. Quantitative spatially resolved measurement of tissue chromophore concentrations using photoacoustic spectroscopy: application to the measurement of blood oxygenation and haemoglobin concentration. Phys. Med. Biol. 52, 141–168 (2007).
Acknowledgements
D.R. acknowledges financial support by the Deutsche Forschungsgemeinschaft (DFG) research grant RA 1848/1-1. M.D. is a fellow of the Studienstiftung des deutschen Volkes. R.W.K. is supported by a BioFuture Award Grant (0311889) of the German Ministry for Education and Research (BMBF). We thank R. Jagasia for providing Hela mitodsRed cells.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Rights and permissions
About this article
Cite this article
Razansky, D., Distel, M., Vinegoni, C. et al. Multispectral opto-acoustic tomography of deep-seated fluorescent proteins in vivo. Nature Photon 3, 412–417 (2009). https://doi.org/10.1038/nphoton.2009.98
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphoton.2009.98
This article is cited by
-
Dual-targeting nanozyme for tumor activatable photo-chemodynamic theranostics
Journal of Nanobiotechnology (2022)
-
Hybrid magnetic resonance and optoacoustic tomography (MROT) for preclinical neuroimaging
Light: Science & Applications (2022)
-
Optical reciprocity induced wavefront shaping for axial and lateral shifting of focus through a scattering medium
Scientific Reports (2022)
-
Multiscale optical and optoacoustic imaging of amyloid-β deposits in mice
Nature Biomedical Engineering (2022)
-
Non-invasive imaging of tau-targeted probe uptake by whole brain multi-spectral optoacoustic tomography
European Journal of Nuclear Medicine and Molecular Imaging (2022)