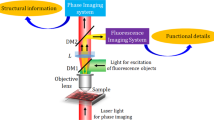
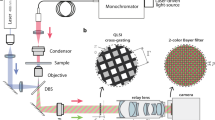
Abstract
Holography is an attractive imaging technique as it offers the ability to view a complete three-dimensional volume from one image. However, holography is not widely applied to the field of three-dimensional fluorescence microscopic imaging, because fluorescence is incoherent and creating holograms requires a coherent interferometer system. Although scanning one beam of an interferometer pattern across the rear aperture of an objective to excite fluorescence in a specimen overcomes the coherence limitation, the mechanical scanning is complicated, which makes the image capturing slow, and the process is limited to low-numerical-aperture objectives. Here we present the first demonstration of a motionless microscopy system (FINCHSCOPE) based on Fresnel incoherent correlation holography, and its use in recording high-resolution three-dimensional fluorescent images of biological specimens. By using high-numerical-aperture objectives, a spatial light modulator, a CCD camera and some simple filters, FINCHSCOPE enables the acquisition of three-dimensional microscopic images without the need for scanning.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gabor, D. A new microscopic principle. Nature 161, 777–778 (1948).
Garcia-Sucerquia, J. et al. Digital in-line holographic microscopy. Appl. Opt. 45, 836–850 (2006).
Poon, T.-C. Scanning holography and two-dimensional image processing by acousto-optic two-pupil syntheses. J. Opt. Soc. Am. A 2, 521–527 (1985).
Schilling, B. W. et al. Three-dimensional holographic fluorescence microscopy. Opt. Lett. 22, 1506–1508 (1997).
Rosen, J. & Brooker, G. Digital spatially incoherent Fresnel holography. Opt. Lett. 32, 912–914 (2007).
Rosen, J. & Brooker, G. Fluorescence incoherent color holography. Opt. Express. 15, 2244–2250 (2007).
Indebetouw, G., Tada, Y., Rosen, J. & Brooker, G. Scanning holographic microscopy with resolution exceeding the Rayleigh limit of the objective by superposition of off-axis holograms. Appl. Opt. 46, 993–1000 (2007).
Gu, M. Principles of Three-Dimensional Imaging in Confocal Microscopes (World Scientific, Singapore 1996).
McNally, J. G., Karpova, T., Cooper, J. & Conchello, J. A. Three-dimensional imaging by deconvolution microscopy. Methods 19, 373–385 (1999).
Cowley, J. M. Off-axis STEM or TEM holography combined with four-dimensional diffraction imaging. Microsc. Microanal. 10, 9–15 (2004).
Goodman, J. W. Introduction to Fourier Optics 2nd edn, 63–95 (McGraw-Hill, New York, 1996).
Acknowledgements
This work was supported by National Science Foundation grant no. 0420382 and CellOptic. We thank M. DeBernardi, B. Storrie and S. Krueger for valuable comments and A. Hermerschmidt, who provided the special firmware for the SLM. We also thank W.R. Kennedy and G. Wendelschager-Crabb for the immunolabelled slide of skin and K. Ryan for deconvolving the stack of images created from the hologram.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
Joseph Rosen and Gary Brooker are the founders of CellOptic and have an equity interest in the company.
Supplementary information
Supplementary Information
Supplementary Information S1 (PDF 77 kb)
Supplementary Information
Supplementary video S1 brookersupfig1.avi (AVI 1508 kb)
Rights and permissions
About this article
Cite this article
Rosen, J., Brooker, G. Non-scanning motionless fluorescence three-dimensional holographic microscopy. Nature Photon 2, 190–195 (2008). https://doi.org/10.1038/nphoton.2007.300
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphoton.2007.300
This article is cited by
-
Deep learning-based incoherent holographic camera enabling acquisition of real-world holograms for holographic streaming system
Nature Communications (2023)
-
High-speed phase-shifting incoherent digital holography (invited)
Applied Physics B (2023)
-
Compensation for reconstructed image distortion using camera model with lens distortion in incoherent digital holography
Optical Review (2022)
-
Roadmap of incoherent digital holography
Applied Physics B (2022)
-
Dual-comb hyperspectral digital holography
Nature Photonics (2021)