Abstract
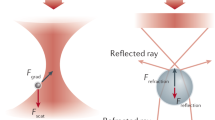
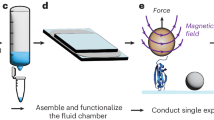
Mass and electrical charge are fundamental properties of biological macromolecules. Although molecular mass has long been determined with atomic precision, a direct and precise determination of molecular charge remains an outstanding challenge. Here we report high-precision (<1e) measurements of the electrical charge of molecules such as nucleic acids, and globular and disordered proteins in solution. The measurement is based on parallel external field-free trapping of single macromolecules, permits the estimation of a dielectric coefficient of the molecular interior and can be performed in real time. Further, we demonstrate the direct detection of single amino acid substitution and chemical modifications in proteins. As the electrical charge of a macromolecule strongly depends on its three-dimensional conformation, this kind of high-precision electrometry offers an approach to probe the structure, fluctuations and interactions of a single molecule in solution.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Perutz, M. F. Electrostatic effects in proteins. Science 201, 1187–1191 (1978).
Warshel, A., Sharma, P. K., Kato, M. & Parson, W. W. Modeling electrostatic effects in proteins. Biochim. Biophys. Acta 1764, 1647–1676 (2006).
Bah, A. et al. Folding of an intrinsically disordered protein by phosphorylation as a regulatory switch. Nature 519, 106–109 (2015).
Kamerlin, S. C. L., Sharma, P. K., Prasad, R. B. & Warshel, A. Why nature really chose phosphate. Q. Rev. Biophys. 46, 1–132 (2013).
Lawrence, M. S., Phillips, K. J. & Liu, D. R. Supercharging proteins can impart unusual resilience. J. Am. Chem. Soc. 129, 10110–10112 (2007).
Mirceta, S. et al. Evolution of mammalian diving capacity traced by myoglobin net surface charge. Science 340, 1234192 (2013).
Martin, L., Latypova, X. & Terro, F. Post-translational modifications of tau protein: implications for Alzheimer's disease. Neurochem. Int. 58, 458–471 (2011).
Bode, A. M. & Dong, Z. G. Post-translational modification of p53 in tumorigenesis. Nat. Rev. Cancer 4, 793–805 (2004).
Manning, G. S. Limiting laws and counterion condensation in polyelectrolyte solutions. I. Colligative properties. J. Chem. Phys. 51, 924–933 (1969).
Netz, R. R. & Orland, H. Variational charge renormalization in charged systems. Eur. Phys. J. E 11, 301–311 (2003).
Lund, M. & Jonsson, B. Charge regulation in biomolecular solution. Q. Rev. Biophys. 46, 265–281 (2013).
Ninham, B. W. & Parsegian, V. A. Electrostatic potential between surfaces bearing ionizable groups in ionic equilibrium with physiologic saline solution. J. Theor. Biol. 31, 405–428 (1971).
Alexander, S. et al. Charge renormalization, osmotic-pressure, and bulk modulus of colloidal crystals—theory. J. Chem. Phys. 80, 5776–5781 (1984).
Aubouy, M., Trizac, E. & Bocquet, L. Effective charge versus bare charge: an analytical estimate for colloids in the infinite dilution limit. J. Phys. A 36, 5835–5840 (2003).
Belloni, L. Ionic condensation and charge renormalization in colloidal suspensions. Colloids Surf. A 140, 227–243 (1998).
Krishnan, M., Mojarad, N., Kukura, P. & Sandoghdar, V. Geometry-induced electrostatic trapping of nanometric objects in a fluid. Nature 467, 692–695 (2010).
Mojarad, N. & Krishnan, M. Measuring the size and charge of single nanoscale objects in solution using an electrostatic fluidic trap. Nat. Nanotech. 7, 448–452 (2012).
Gast, K. et al. Prothymosin-alpha—a biologically-active protein with random coil conformation. Biochemistry 34, 13211–13218 (1995).
Mueller-Spaeth, S. et al. Charge interactions can dominate the dimensions of intrinsically disordered proteins. Proc. Natl Acad. Sci. USA 107, 14609–14614 (2010).
Rozycka, M. et al. Intrinsically disordered and pliable starmaker-like protein from Medaka (Oryzias latipes) controls the formation of calcium carbonate crystals. PLoS ONE 9, e114308 (2014).
Kramers, H. A. Brownian motion in a field of force and the diffusion model of chemical reactions. Physica 7, 284–304 (1940).
Krishnan, M. Electrostatic free energy for a confined nanoscale object in a fluid. J. Chem. Phys. 138, 114906 (2013).
Manning, G. S. Molecular theory of polyelectrolyte solutions with applications to electrostatic properties of polynucleotides. Q. Rev. Biophys. 11, 179–246 (1978).
Manning, G. S. Approximate solutions to some problems in polyelectrolyte theory involving nonuniform charge distributions. Macromolecules 41, 6217–6227 (2008).
Gordon, J. C. et al. H++: a server for estimating pKas and adding missing hydrogens to macromolecules. Nucleic Acids Res. 33, W368–W371 (2005).
Li, L., Li, C., Zhang, Z. & Alexov, E. On the ‘dielectric constant’ of proteins: smooth dielectric function for macromolecular modeling and its implementation in DelPhi. J. Chem. Theory Comput. 9, 2126–2136 (2013).
Sham, Y. Y., Muegge, I. & Warshel, N. Effect of protein relaxation on charge–charge interaction and dielectric constants in protein. Biophys. J. 76, A198–A198 (1999).
Isom, D. G., Castaneda, C. A., Cannon, B. R. & Garcia-Moreno, B. E. Large shifts in pKa values of lysine residues buried inside a protein. Proc. Natl Acad. Sci. USA 108, 5260–5265 (2011).
Piliarik, M. & Sandoghdar, V. Direct optical sensing of single unlabelled proteins and super-resolution imaging of their binding sites. Nat. Commun. 5, 4495 (2014).
Arroyo, J. O. et al. Label-free, all-optical detection, imaging, and tracking of a single protein. Nano Lett. 14, 2065–2070 (2014).
Wang, Q. & Moerner, W. E. Single-molecule motions enable direct visualization of biomolecular interactions in solution. Nat. Methods 11, 555–558 (2014).
Gao, J. M., Mammen, M. & Whitesides, G. M. Evaluating electrostatic contributions to binding with the use of protein charge ladders. Science 272, 535–537 (1996).
Winston, R. L. & Fitzgerald, M. C. Mass spectrometry as a readout of protein structure and function. Mass Spectrom. Rev. 16, 165–179 (1997).
Sharon, M. & Robinson, C. V. The role of mass spectrometry in structure elucidation of dynamic protein complexes. Annu. Rev. Biochem. 76, 167–193 (2007).
Acknowledgements
We acknowledge M. Borgia for the generous gift of unlabelled ProTαC protein, D. Nettels for help with fluorescence correlation spectroscopy (FCS) instrumentation and providing FCS data-analysis software, S. Chesnov from the Functional Genomics Center Zurich for mass spectrometry analysis, A. Caflisch, A. Soranno, G. Manning and R. Netz for discussions, and the Swiss National Science Foundation and University of Zurich for financial support. Nanofabrication was carried out at the FIRST Center for Micro- and Nanoscience, ETH Zurich.
Author information
Authors and Affiliations
Contributions
F.R. performed the experiments and analysed the data. F.Z. and F.R. purified and labelled the proteins and nucleic acids and performed and analysed the FCS experiments. N.M., M.R., M.W. and A.O. provided the unlabelled Stm-l protein. F.Z. and B.S. contributed biochemical expertise, including protein selection. M.K. conceived the project, performed the theoretical analysis, interpreted the data and wrote the manuscript. F.R., F.Z. and B.S. provided feedback on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4695 kb)
Supplementary information
Supplementary Movie 1 (AVI 1504 kb)
Supplementary information
Supplementary Movie 2 (AVI 842 kb)
Rights and permissions
About this article
Cite this article
Ruggeri, F., Zosel, F., Mutter, N. et al. Single-molecule electrometry. Nature Nanotech 12, 488–495 (2017). https://doi.org/10.1038/nnano.2017.26
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2017.26
This article is cited by
-
Stable trapping of multiple proteins at physiological conditions using nanoscale chambers with macromolecular gates
Nature Communications (2023)
-
Nanopore electro-osmotic trap for the label-free study of single proteins and their conformations
Nature Nanotechnology (2021)
-
Glossary on atmospheric electricity and its effects on biology
International Journal of Biometeorology (2021)
-
Optical imaging of single-protein size, charge, mobility, and binding
Nature Communications (2020)
-
Size and surface charge characterization of nanoparticles with a salt gradient
Nature Communications (2020)