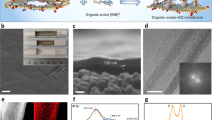
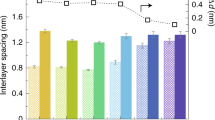
Abstract
Carbon nanomaterials are robust and possess fascinating properties useful for separation technology applications, but their scalability and high salt rejection when in a strong cross flow for long periods of time remain challenging. Here, we present a graphene-based membrane that is prepared using a simple and environmentally friendly method by spray coating an aqueous dispersion of graphene oxide/few-layered graphene/deoxycholate. The membranes were robust enough to withstand strong cross-flow shear for a prolonged period (120 h) while maintaining NaCl rejection near 85% and 96% for an anionic dye. Experimental results and molecular dynamic simulations revealed that the presence of deoxycholate enhances NaCl rejection in these graphene-based membranes. In addition, these novel hybrid-layered membranes exhibit better chlorine resistance than pure graphene oxide membranes. The desalination performance and aggressive shear and chlorine resistance of these scalable graphene-based membranes are promising for use in practical water separation applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mohammad, A. W. et al. Nanofiltration membranes review: recent advances and future prospects. Desalination 356, 226–254 (2015).
Lee, C., Wei, X., Kysar, J. W. & Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321, 385–388 (2008).
Cohen-Tanugi, D. & Grossman, J. C. Mechanical strength of nanoporous graphene as a desalination membrane. Nano Lett. 14, 6171–6178 (2014).
Cohen-Tanugi, D. & Grossman, J. C. Water desalination across nanoporous graphene. Nano Lett. 12, 3602–3608 (2012).
Surwade, S. P. et al. Water desalination using nanoporous single-layer graphene. Nat. Nanotech. 10, 459–464 (2015).
Tölle, F. J., Gamp, K. & Mülhaupt, R. Scale-up and purification of graphite oxide as intermediate for functionalized graphene. Carbon 75, 432–442 (2014).
Segal, M. Selling graphene by the ton. Nat. Nanotech. 4, 612–614 (2009).
Raidongia, K. & Huang, J. Nanofluidic ion transport through reconstructed layered materials. J. Am. Chem. Soc. 134, 16528–16531 (2012).
Cruz-Silva, R., Endo, M. & Terrones, M. Graphene oxide films, fibers, and membranes. Nanotechnol. Rev. 5, 377–391 (2016).
Han, Y., Xu, Z. & Gao, C. Ultrathin graphene nanofiltration membrane for water purification. Adv. Funct. Mater. 23, 3693–3700 (2013).
Goh, K. et al. All-carbon nanoarchitectures as high-performance separation membranes with superior stability. Adv. Funct. Mater. 25, 7348–7359 (2015).
Huang, H. et al. Ultrafast viscous water flow through nanostrand-channelled graphene oxide membranes. Nat. Commun. 4, 2979 (2013).
Akbari, A . et al. Large-area graphene-based nanofiltration membranes by shear alignment of discotic nematic liquid crystals of graphene oxide. Nat. Commun. 7, 10891 (2016).
Abraham, J . et al. Tunable sieving of ions using graphene oxide membranes. Nat. Nanotech. 12, 546–550 (2017).
Yeh, C., Raidongia, K., Shao, J., Yang, Q. & Huang, J. On the origin of the stability of graphene oxide membranes in water. Nat. Chem. 7, 166–170 (2015).
Park, S. et al. Graphene oxide papers modified by divalent ions—enhancing mechanical properties via chemical cross-linking. ACS Nano 2, 572–578 (2008).
Endo, M. et al. Nanotechnology: ‘buckypaper’ from coaxial nanotubes. Nature 433, 476 (2005).
Salavagione, H. J., Gomez, M. A. & Martinez, G. Polymeric modification of graphene through esterification of graphite oxide and poly(vinyl alcohol). Macromolecules 42, 6331–6334 (2009).
Nicolaï, A., Sumpter, B. G. & Meunier, V. Tunable water desalination across graphene oxide framework membranes. Phys. Chem. Chem. Phys. 16, 8646–8654 (2014).
Baker, R. W. Membrane Technology and Applications (Wiley, 2012).
Wei, Y. et al. Declining flux and narrowing nanochannels under wrinkles of compacted graphene oxide nanofiltration membranes. Carbon 108, 568–575 (2016).
Hu, M. & Mi, B. Enabling graphene oxide nanosheets as water separation membranes. Environ. Sci. Technol. 47, 3715–3723 (2013).
Han, Y., Jiang, Y. & Gao, C. High-flux graphene oxide nanofiltration membrane intercalated by carbon nanotubes. ACS Appl. Mater. Interfaces 7, 8147–8155 (2015).
Chan, W. et al. Zwitterion functionalized carbon nanotube/polyamide nanocomposite. ACS Nano 7, 5308–5319 (2013).
Selectivity, M.-M. I. et al. Ion-responsive channels of zwitterion-carbon nanotube membrane for rapid water permeation and ultrahigh. ACS Nano 9, 7488–7496 (2015).
Liu, G. et al. Graphene oxide for high-efficiency separation membranes: role of electrostatic interactions. Carbon 110, 56–61 (2016).
Afonso, M. D., Hagmeyer, G. & Gimbel, R. Streaming potential measurements to assess the variation of nanofiltration membranes surface charge with the concentration of salt solutions. Sep. Purif. Technol. 22–23, 52–541 (2001).
Bartels, C., Franks, R., Rybar, S. & Schierach, M. The effect of feed ionic strength on salt passage through reverse osmosis membranes. Desalination 184, 185–195 (2005).
Inukai, S. et al. High-performance multi-functional reverse osmosis membranes obtained by carbon nanotube·polyamide nanocomposite. Sci. Rep. 5, 13562 (2015).
Wei, N., Peng, X. & Xu, Z. Understanding water permeation in graphene oxide membranes. Appl. Mater. Interfaces 6, 5877–5883 (2014).
Boukhvalov, D. W., Katsnelson, M. I. & Son, Y.-W. Origin of anomalous water permeation through graphene oxide membrane. Nano Lett. 13, 3930–3935 (2013).
Mallapragada, S. K. & Peppas, N. A. Dissolution mechanism of semicrystalline poly(vinyl alcohol) in water. J. Polym. Sci. 34, 1339–1346 (1996).
Marcano, D. C. et al. Improved synthesis of graphene oxide. ACS Nano 4, 4806–4814 (2010).
Cai, W. et al. Synthesis and solid-state NMR structural characterization of 13C-labeled graphite oxide. Science 321, 1815–1817 (2008).
Smith, W. & Todorov, I. T. A short description of DL_POLY. Mol. Simul. 32, 935–943 (2006).
Mayo, S. L., Olafson, B. D. & Goddard, W. A. DREIDING: a generic force field for molecular simulations. J. Phys. Chem. 101, 8897–8909 (1990).
Valiev, M. et al. NWChem: a comprehensive and scalable open-source solution for large scale molecular simulations. Comput. Phys. Commun. 181, 1477–1489 (2010).
Humphrey, W., Dalke, A. & Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 14, 33–38 (1996).
Kusalik, P. G. & Svishchev, I. M. The spatial structure in liquid water. Science 265, 1219–1221 (1994).
Acknowledgements
This work was supported by the Center of Innovation Program, Global Aqua Innovation Center for Improving Living Standards and Water Sustainability, from the Japan Science and Technology Agency (JST).
Author information
Authors and Affiliations
Contributions
A.M.-G. designed the experiments, performed FTIR, SEM, Raman spectroscopy and desalination, and wrote the manuscript. R.C.-S. carried out GO synthesis and XPS, and wrote the manuscript. H.M. performed discussion and preliminary DWCNT samples. J.O.-M. performed XRD and wrote the manuscript. T.A. and S.T. performed molecular dynamic simulations. T.F. provided valuable technical assistance. T.H. performed TEM observations. K.T., M.T. and M.E. participated in discussions and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary Information (PDF 4914 kb)
Supplementary Movie 1
Supplementary Movie 1 (MP4 39868 kb)
Supplementary Movie 2
Supplementary Movie 2 (MP4 40210 kb)
Supplementary Movie 3
Supplementary Movie 3 (MP4 38708 kb)
Supplementary Movie 4
Supplementary Movie 4 (MP4 38550 kb)
Rights and permissions
About this article
Cite this article
Morelos-Gomez, A., Cruz-Silva, R., Muramatsu, H. et al. Effective NaCl and dye rejection of hybrid graphene oxide/graphene layered membranes. Nature Nanotech 12, 1083–1088 (2017). https://doi.org/10.1038/nnano.2017.160
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2017.160
This article is cited by
-
Nanowire-assisted electrochemical perforation of graphene oxide nanosheets for molecular separation
Nature Communications (2024)
-
Graphene oxide-based membranes for water desalination and purification
npj 2D Materials and Applications (2024)
-
High-surface-area functionalized nanolaminated membranes for energy-efficient nanofiltration and desalination in forward osmosis
Nature Water (2023)
-
Anomalous water molecular gating from atomic-scale graphene capillaries for precise and ultrafast molecular sieving
Nature Communications (2023)
-
Graphene oxide for photonics, electronics and optoelectronics
Nature Reviews Chemistry (2023)