Abstract
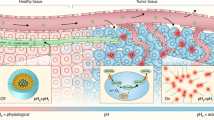
Engineered nanoparticles that respond to pathophysiological parameters, such as pH or redox potential, have been developed as contrast agents for the magnetic resonance imaging (MRI) of tumours. However, beyond anatomic assessment, contrast agents that can sense these pathological parameters and rapidly amplify their magnetic resonance signals are desirable because they could potentially be used to monitor the biological processes of tumours and improve cancer diagnosis. Here, we report an MRI contrast agent that rapidly amplifies magnetic resonance signals in response to pH. We confined Mn2+ within pH-sensitive calcium phosphate (CaP) nanoparticles comprising a poly(ethylene glycol) shell. At a low pH, such as in solid tumours, the CaP disintegrates and releases Mn2+ ions. Binding to proteins increases the relaxivity of Mn2+ and enhances the contrast. We show that these nanoparticles could rapidly and selectively brighten solid tumours, identify hypoxic regions within the tumour mass and detect invisible millimetre-sized metastatic tumours in the liver.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Na, H. B. & Hyeon, T. Nanostructured T1 MRI contrast agents. J. Mater. Chem. 19, 6267–6273 (2009).
Sun, C., Lee, J. S. H. & Zhang, M. Q. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliver. Rev. 60, 1252–1265 (2008).
Ghosh, D. et al. M13-templated magnetic nanoparticles for targeted in vivo imaging of prostate cancer. Nature Nanotech. 7, 677–682 (2012).
Lee, J. H. et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nature Med. 13, 95–99 (2007).
Burrell, R. A., McGranahan, N., Bartek, J. & Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 501, 338–345 (2013).
Kim, T. et al. Urchin-shaped manganese oxide nanoparticles as pH-responsive activatable T1 contrast agents for magnetic resonance imaging. Angew. Chem. Int. Ed. 50, 10589–10593 (2011).
Gao, G. H. et al. Magnetite-nanoparticle-encapsulated pH-responsive polymeric micelle as an MRI probe for detecting acidic pathologic areas. Small 6, 1201–1204 (2010).
Chen, Y. et al. Manganese oxide-based multifunctionalized mesoporous silica nanoparticles for pH-responsive MRI, ultrasonography and circumvention of MDR in cancer cells. Biomaterials 33, 7126–7137 (2012).
Sowers, M. A. et al. Redox-responsive branched-bottlebrush polymers for in vivo MRI and fluorescence imaging. Nature Commun. 5, 5460 (2014).
Huang, X. N. et al. Multi-chromatic pH-activatable 19F-MRI nanoprobes with binary ON/OFF pH transitions and chemical-shift barcodes. Angew. Chem. Int. Ed. 52, 8074–8078 (2013).
Oishi, M., Sumitani, S. & Nagasaki, Y. On–Off regulation of 19F magnetic resonance signals based on pH-sensitive PEGylated nanogels for potential tumor-specific smart 19F MRI probes. Bioconjug. Chem. 18, 1379–1382 (2007).
Bennewitz, M. F. et al. Biocompatible and pH-sensitive PLGA encapsulated MnO nanocrystals for molecular and cellular MRI. ACS Nano 5, 3438–3446 (2011).
Koylu, M. Z., Asubay, S. & Yilmaz, A. Determination of proton relaxivities of Mn(II), Cu(II) and Cr(III) added to solutions of serum proteins. Molecules 14, 1537–1545 (2009).
Aime, S., Canton, S., Crich, S. G. & Terreno, E. 1H and 17O relaxometric investigations of the binding of Mn(II) ion to human serum albumin. Magn. Reson. Chem. 40, 41–48 (2002).
Na, H. B. et al. Development of a T1 contrast agent for magnetic resonance imaging using MnO nanoparticles. Angew. Chem. Int. Ed. 46, 5397–5401 (2007).
Zhang, M. Z. & Kataoka, K. Nano-structured composites based on calcium phosphate for cellular delivery of therapeutic and diagnostic agents. Nano Today 4, 508–517 (2009).
LeGeros, R. Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 108, 4742–4753 (2008).
Kakizawa, Y. & Kataoka, K. Block copolymer self-assembly into monodispersive nanoparticles with hybrid core of antisense DNA and calcium phosphate. Langmuir 18, 4539–4543 (2002).
Yushmanov, V. E., Tominaga, T. T., Borissevitch, I. E., Imasato, H. & Tabak, M. Binding of manganese and iron tetraphenylporphine sulfonates to albumin is relevant to their contrast properties. Magn. Reson. Imaging 14, 255–261 (1996).
Marchal, G. et al. Comparison between Gd-DTPA, Gd-EOB-DTPA, and Mn-DPDP in induced HCC in rats: a correlation study of MR imaging, microangiography, and histology. Magn Reson Imaging 11, 665–674 (1993).
Wheaton, A. J. & Miyazaki, M. Non-contrast enhanced MR angiography: physical principles. J. Magn. Reson. Imaging 36, 286–304 (2012).
Terreno, E., Castelli, D. D., Viale, A. & Aime, S. Challenges for molecular magnetic resonance imaging. Chem. Rev. 110, 3019–3042 (2010).
Gatenby, R. A. & Gillies, R. J. Why do cancers have high aerobic glycolysis? Nature Rev. Cancer 4, 891–899 (2004).
Helmlinger, G., Yuan, F., Dellian, M. & Jain, R. K. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nature Med. 3, 177–182 (1997).
Gujar, S. K., Maheshwari, S., Bjorkman-Burtscher, I. & Sundgren, P. C. Magnetic resonance spectroscopy. J. Neuroophthalmol. 25, 217–226 (2005).
Fingar, V. H., Wieman, T. J., Park, Y. J. & Henderson, B. W. Implications of a pre-existing tumor hypoxic fraction on photodynamic therapy. J. Surg. Res. 53, 524–528 (1992).
Vogl, T. J. et al. Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology 200, 59–67 (1996).
Cory, D. A., Schwartzentruber, D. J. & Mock, B. H. Ingested manganese chloride as a contrast agent for magnetic resonance imaging. Magn. Reson. Imaging 5, 65–70 (1987).
Wu, H. L. et al. Polymeric micelles loaded with platinum anticancer drugs target preangiogenic micrometastatic niches associated with inflammation. J. Control. Release 189, 1–10 (2014).
Lu, H. T., Ouyang, W. M. & Huang, C. S. Inflammation, a key event in cancer development. Mol. Cancer Res. 4, 221–233 (2006).
Gallagher, F. A. et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature 453, 940–943 (2008).
Webb, B. A., Chimenti, M., Jacobson, M. P. & Barber, D. L. Dysregulated pH: a perfect storm for cancer progression. Nature Rev. Cancer 11, 671–677 (2011).
Wilson, W. R. & Hay, M. P. Targeting hypoxia in cancer therapy. Nature Rev. Cancer 11, 393–410 (2011).
Hashim, A. I., Zhang, X. M., Wojtkowiak, J. W., Martinez, G. V. & Gillies, R. J. Imaging pH and metastasis. NMR Biomed. 24, 582–591 (2011).
Zhang, G., Palmer, G. M., Dewhirst, M. W. & Fraser, C. L. A dual-emissive-materials design concept enables tumour hypoxia imaging. Nature Mater. 8, 747–751 (2009).
Kircher, M. F. et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nature Med. 18, 829–834 (2012).
Komar, G. et al. 18F-EF5: a new PET tracer for imaging hypoxia in head and neck cancer. J. Nucl. Med. 49, 1944–1951 (2008).
Schroeder, A. et al. Treating metastatic cancer with nanotechnology. Nature Rev. Cancer 12, 39–50 (2012).
Chaffer, C. L. & Weinberg, R. A. A perspective on cancer cell metastasis. Science 331, 1559–1564 (2011).
Ichikawa, T. et al. Detection and characterization of focal liver lesions: a Japanese phase III, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominantly in patients with hepatocellular carcinoma and chronic liver disease. Invest. Radiol. 45, 133–141 (2010).
Chan, K. W. Y. et al. MRI-detectable pH nanosensors incorporated into hydrogels for in vivo sensing of transplanted-cell viability. Nature Mater. 12, 268–275 (2013).
Willmann, J. K., van Bruggen, N., Dinkelborg, L. M. & Gambhir, S. S. Molecular imaging in drug development. Nature Rev. Drug Discov. 7, 591–607 (2008).
Weissleder, R. & Pittet, M. J. Imaging in the era of molecular oncology. Nature 452, 580–589 (2008).
Nishiyama, N. et al. Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res. 63, 8977–8983 (2003).
Cabral, H. et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nature Nanotechnol. 6, 815–823 (2011).
Terada, Y. et al. Construction and commissioning of BL37XU at SPring8. AIP Conf. Proc. 705, 376–379 (2004).
Acknowledgements
This research was financially supported by the Center of Innovation Program stream from the Japan Science and Technology Agency and the Funding Program for World-Leading Innovative R&D on Science and Technology from the Japan Society for the Promotion of Science to K.K. The TEM characterization at the Research Hub for Advanced Nano Characterization, The University of Tokyo, was supported by the Ministry of Education, Culture, Sports, Science and Technology, Japan. The μ-SR-XRF characterization was supported by the Nanotechnology Support Program of the Japan Synchrotron Radiation Research Institute. We thank S. Shibata, N. Nitta, Y. Ozawa, S. Murayama and A. Sekita for assistance with MRI experiments.
Author information
Authors and Affiliations
Contributions
P.M. designed and performed the experiments. D.K. and I.A. performed MRI measurements. H.C. and Y.T. helped in the μ-SR-XRF measurement. H.W. performed immunostaining. P.M., D.K., H.C., I.A., N.N., T.S. and K.K. discussed and analysed the data. P.M. wrote and revised the manuscript. H.C., I.A., N.N. and K.K. commented and revised the manuscript. H.C. N.N., T.S., I.A. and K.K. led or supervised this project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4528 kb)
Supplementary information
Supplementary Movie 1 (MOV 2005 kb)
Supplementary information
Supplementary Movie 2 (MOV 3149 kb)
Rights and permissions
About this article
Cite this article
Mi, P., Kokuryo, D., Cabral, H. et al. A pH-activatable nanoparticle with signal-amplification capabilities for non-invasive imaging of tumour malignancy. Nature Nanotech 11, 724–730 (2016). https://doi.org/10.1038/nnano.2016.72
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2016.72