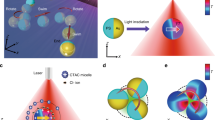
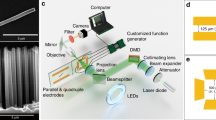
Abstract
Phototaxis is commonly observed in motile photosynthetic microorganisms. For example, green algae are capable of swimming towards a light source (positive phototaxis) to receive more energy for photosynthesis, or away from a light source (negative phototaxis) to avoid radiation damage or to hide from predators. Recently, with the aim of applying nanoscale machinery to biomedical applications, various inorganic nanomotors based on different propulsion mechanisms have been demonstrated. The only method to control the direction of motion of these self-propelled micro/nanomotors is to incorporate a ferromagnetic material into their structure and use an external magnetic field for steering. Here, we show an artificial microswimmer that can sense and orient to the illumination direction of an external light source. Our microswimmer is a Janus nanotree containing a nanostructured photocathode and photoanode at opposite ends that release cations and anions, respectively, propelling the microswimmer by self-electrophoresis. Using chemical modifications, we can control the zeta potential of the photoanode and program the microswimmer to exhibit either positive or negative phototaxis. Finally, we show that a school of microswimmers mimics the collective phototactic behaviour of green algae in solution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Nultsch, W. in Primitive Sensory and Communication Systems. The Taxis and Tropism of Microorganisms and Cells (ed. Carlile, M. J.) 29–90 (Academic Press, 1975).
Jékely, G. Evolution of phototaxis. Phil. Trans. R Soc. B 364, 2795–2808 (2009).
Jekely, G. et al. Mechanism of phototaxis in marine zooplankton. Nature 456, 395–399 (2008).
Weibel, D. B. et al. Microoxen: microorganisms to move microscale loads. Proc. Natl Acad. Sci. USA 102, 11963–11967 (2005).
Paxton, W. F. et al. Catalytic nanomotors: autonomous movement of striped nanorods. J. Am. Chem. Soc. 126, 13424–13431 (2004).
Paxton, W. F. et al. Catalytically induced electrokinetics for motors and micropumps. J. Am. Chem. Soc. 128, 14881–14888 (2006).
Guix, M., Mayorga-Martinez, C. C. & Merkoci, A. Nano/micromotors in (bio)chemical science applications. Chem. Rev. 114, 6285–6322 (2014).
Wang, W., Duan, W. T., Ahmed, S., Mallouk, T. E. & Sen, A. Small power: autonomous nano- and micromotors propelled by self-generated gradients. Nano Today 8, 531–554 (2013).
Ma, X., Hahn, K. & Sanchez, S. Catalytic mesoporous Janus nanomotors for active cargo delivery. J. Am. Chem. Soc. 137, 4976–4979 (2015).
Magdanz, V., Stoychev, G., Ionov, L., Sanchez, S. & Schmidt, O. G. Stimuli-responsive microjets with reconfigurable shape. Angew. Chem. Int. Ed. 53, 2673–2677 (2014).
Wilson, D. A., Nolte, R. J. & van Hest, J. C. Autonomous movement of platinum-loaded stomatocytes. Nat. Chem. 4, 268–274 (2012).
Hong, Y. Y., Diaz, M., Cordova-Figueroa, U. M. & Sen, A. Light-driven titanium-dioxide-based reversible microfireworks and micromotor/micropump systems. Adv. Funct. Mater. 20, 1568–1576 (2010).
Giudicatti, S. et al. Photoactive rolled-up TiO2 microtubes: fabrication, characterization and applications. J. Mater. Chem. C 2, 5892–5901 (2014).
Palagi, S. et al. Structured light enables biomimetic swimming and versatile locomotion of photoresponsive soft microrobots. Nat. Mater. 15, 647–653 (2016).
Wang, W. et al. Acoustic propulsion of nanorod motors inside living cells. Angew. Chem. Int. Ed. 53, 3201–3204 (2014).
Walker, D., Kasdorf, B. T., Jeong, H. H., Lieleg, O. & Fischer, P. Enzymatically active biomimetic micropropellers for the penetration of mucin gels. Sci. Adv. 1, e1500501 (2015).
Loget, G. & Kuhn, A. Electric field-induced chemical locomotion of conducting objects. Nat. Commun. 2, 535 (2011).
Gao, W. & Wang, J. Synthetic micro/nanomotors in drug delivery. Nanoscale 6, 10486–10494 (2014).
Kagan, D. et al. Acoustic droplet vaporization and propulsion of perfluorocarbon-loaded microbullets for targeted tissue penetration and deformation. Angew. Chem. Int. Ed. 51, 7519–7522 (2012).
Mino, G. et al. Enhanced diffusion due to active swimmers at a solid surface. Phys. Rev. Lett. 106, 048102 (2011).
Hong, Y., Blackman, N. M., Kopp, N. D., Sen, A. & Velegol, D. Chemotaxis of nonbiological colloidal rods. Phys. Rev. Lett. 99, 178103 (2007).
Baraban, L., Harazim, S. M., Sanchez, S. & Schmidt, O. G. Chemotactic behavior of catalytic motors in microfluidic channels. Angew. Chem. Int. Ed. 52, 5552–5556 (2013).
Saha, S., Golestanian, R. & Ramaswamy, S. Clusters, asters, and collective oscillations in chemotactic colloids. Phys. Rev. E 89, 062316 (2014).
Dey, K. K. et al. Micromotors powered by enzyme catalysis. Nano Lett. 15, 8311–8315 (2015).
Peng, F., Tu, Y., van Hest, J. C. & Wilson, D. A. Self-guided supramolecular cargo-loaded nanomotors with chemotactic behavior towards cells. Angew. Chem. Int. Ed. 54, 11662–11665 (2015).
Dey, K. K., Bhandari, S., Bandyopadhyay, D., Basu, S. & Chattopadhyay, A. The pH taxis of an intelligent catalytic microbot. Small 9, 1916–1920 (2013).
Solovev, A. A., Sanchez, S., Pumera, M., Mei, Y. F. & Schmidt, O. G. Magnetic control of tubular catalytic microbots for the transport, assembly, and delivery of micro-objects. Adv. Funct. Mater. 20, 2430–2435 (2010).
Baraban, L. et al. Catalytic Janus motors on microfluidic chip: deterministic motion for targeted cargo delivery. ACS Nano 6, 3383–3389 (2012).
Chaturvedi, N., Hong, Y., Sen, A. & Velegol, D. Magnetic enhancement of phototaxing catalytic motors. Langmuir 26, 6308–6313 (2010).
Ibele, M., Mallouk, T. E. & Sen, A. Schooling behavior of light-powered autonomous micromotors in water. Angew. Chem. Int. Ed. 48, 3308–3312 (2009).
Solovev, A. A., Smith, E. J., Bof’ Bufon, C. C., Sanchez, S. & Schmidt, O. G. Light-controlled propulsion of catalytic microengines. Angew. Chem. Int. Ed. 50, 10875–10878 (2011).
Liu, C., Tang, J., Chen, H. M., Liu, B. & Yang, P. A fully integrated nanosystem of semiconductor nanowires for direct solar water splitting. Nano Lett. 13, 2989–2992 (2013).
Liu, C., Hwang, Y. J., Jeong, H. E. & Yang, P. Light-induced charge transport within a single asymmetric nanowire. Nano Lett. 11, 3755–3758 (2011).
Li, J. et al. Nanomotor lithography. Nat. Commun. 5, 5026 (2014).
Cheng, M., Yang, X., Zhang, F., Zhao, J. & Sun, L. Efficient dye-sensitized solar cells based on hydroquinone/benzoquinone as a bioinspired redox couple. Angew. Chem. Int. Ed. 51, 9896–9899 (2012).
Liu, R. & Sen, A. Autonomous nanomotor based on copper-platinum segmented nanobattery. J. Am. Chem. Soc. 133, 20064–20067 (2011).
Harris, L. B. Simplified calculation of electrophoretic mobility of non-spherical particles when the electrical double layer is very extended. J. Colloid Interface Sci. 34, 322–325 (1970).
Shyue, J. J. et al. Acid–base properties and zeta potentials of self-assembled monolayers obtained via in situ transformations. Langmuir 20, 8693–8698 (2004).
Hwang, Y. J., Hahn, C., Liu, B. & Yang, P. Photoelectrochemical properties of TiO2 nanowire arrays: a study of the dependence on length and atomic layer deposition coating. ACS Nano 6, 5060–5069 (2012).
Triplett, M. et al. Long minority carrier diffusion lengths in bridged silicon nanowires. Nano Lett. 15, 523–529 (2015).
Wakabayashi, K., Misawa, Y., Mochiji, S. & Kamiya, R. Reduction–oxidation poise regulates the sign of phototaxis in Chlamydomonas reinhardtii. Proc. Natl Acad. Sci. USA 108, 11280–11284 (2011).
Nultsch, W., Schuchart, H. & Hohl, M. Investigations on the phototactic orientation of Anabaena variabilis. Arch. Microbiol. 122, 85–91 (1979).
Diehn, B. Phototaxis and sensory transduction in Euglena. Science 181, 1009–1015 (1973).
Horstmeyer, R., Ruan, H. & Yang, C. Guidestar-assisted wavefront-shaping methods for focusing light into biological tissue. Nat. Photon. 9, 563–571 (2015).
Acknowledgements
The authors thank S. Brittman from AMOLF and C. Liu from Harvard University for discussions. This work was supported in part by the Hong Kong Research Grants Council (RGC) General Research Fund (GRF17303015, ECS27300814), the University Grant Council (contract no. AoE/P-04/08), the URC Strategic Research Theme on New Materials and the URC Strategic Research Theme on Clean Energy (University of Hong Kong).
Author information
Authors and Affiliations
Contributions
B.D. and J.T. conceived and designed the experiments. B.D., J.W., Z.X., X.Z. and W.D. fabricated the devices and performed the measurements. C.-C.L. and S.-P.F. helped with zeta potential measurements. B.D. and J.T. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1362 kb)
Supplementary information
Supplementary Movie 1 (MOV 379 kb)
Supplementary information
Supplementary Movie 2 (MOV 12295 kb)
Supplementary information
Supplementary Movie 3 (MOV 2051 kb)
Supplementary information
Supplementary Movie 4 (MOV 1212 kb)
Supplementary information
Supplementary Movie 5 (MOV 3869 kb)
Supplementary information
Supplementary Movie 6 (MOV 9321 kb)
Supplementary information
Supplementary Movie 7 (MOV 20543 kb)
Rights and permissions
About this article
Cite this article
Dai, B., Wang, J., Xiong, Z. et al. Programmable artificial phototactic microswimmer. Nature Nanotech 11, 1087–1092 (2016). https://doi.org/10.1038/nnano.2016.187
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2016.187
This article is cited by
-
Materials consideration for the design, fabrication and operation of microscale robots
Nature Reviews Materials (2024)
-
Succulent-like light-driven micromotor for dynamic fluorescence detection and photocatalytic degradation of tetracycline
Science China Materials (2024)
-
Hydrodynamic pursuit by cognitive self-steering microswimmers
Communications Physics (2023)
-
Smart micro- and nanorobots for water purification
Nature Reviews Bioengineering (2023)
-
Photochromism from wavelength-selective colloidal phase segregation
Nature (2023)