Abstract
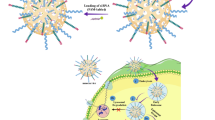
The design of cancer-targeting particles with precisely tuned physicochemical properties may enhance the delivery of therapeutics and access to pharmacological targets. However, a molecular-level understanding of the interactions driving the fate of nanomedicine in biological systems remains elusive. Here, we show that ultrasmall (<10 nm in diameter) poly(ethylene glycol)-coated silica nanoparticles, functionalized with melanoma-targeting peptides, can induce a form of programmed cell death known as ferroptosis in starved cancer cells and cancer-bearing mice. Tumour xenografts in mice intravenously injected with nanoparticles using a high-dose multiple injection scheme exhibit reduced growth or regression, in a manner that is reversed by the pharmacological inhibitor of ferroptosis, liproxstatin-1. These data demonstrate that ferroptosis can be targeted by ultrasmall silica nanoparticles and may have therapeutic potential.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Davis, M. E., Chen, Z. G. & Shin, D. M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 7, 771–782 (2008).
Phillips, E. et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 6, 260ra149 (2014).
Duncan, R. & Richardson, S. C. Endocytosis and intracellular trafficking as gateways for nanomedicine delivery: opportunities and challenges. Mol. Pharmacol. 9, 2380–2402 (2012).
Duncan, R. & Gaspar, R. Nanomedicine(s) under the microscope. Mol. Pharmacol. 8, 2101–2141 (2011).
Ma, X. et al. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano 5, 8629–8639 (2011).
Li, J. J., Hartono, D., Ong, C. N., Bay, B. H. & Yung, L. Y. Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials 31, 5996–6003 (2010).
Chen, N. et al. Long-term effects of nanoparticles on nutrition and metabolism. Small 10, 3603–3611 (2014).
Li, C. et al. PAMAM nanoparticles promote acute lung injury by inducing autophagic cell death through the Akt-TSC2-mTOR signaling pathway. J. Mol. Cell Biol. 1, 37–45 (2009).
Stern, S. T., Adiseshaiah, P. P. & Crist, R. M. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part. Fibre Toxicol. 9, 20 (2012).
Ma, K. et al. Control of ultrasmall sub-10 nm ligand-functionalized fluorescent core–shell silica nanoparticle growth in water. Chem. Mater. 27, 4119–4133 (2015).
Bradbury, M. S. et al. Clinically-translated silica nanoparticles as dual-modality cancer-targeted probes for image-guided surgery and interventions. Integr. Biol. (Camb.) 5, 74–86 (2013).
Benezra, M. et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Invest. 121, 2768–2780 (2011).
Yoo, B. et al. Ultrasmall dual-modality silica nanoparticle drug conjugates: design, synthesis, and characterization. Bioorg. Med. Chem. 23, 7119–7130 (2015).
Miao, Y., Benwell, K. & Quinn, T. P. 99mTc- and 111In-labeled alpha-melanocyte-stimulating hormone peptides as imaging probes for primary and pulmonary metastatic melanoma detection. J. Nucl. Med. 48, 73–80 (2007).
Mizushima, N., Yoshimori, T. & Levine, B. Methods in mammalian autophagy research. Cell 140, 313–326 (2010).
Nelson, D. A. et al. Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes Dev. 18, 2095–2107 (2004).
Kandasamy, K. et al. Involvement of proapoptotic molecules Bax and Bak in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced mitochondrial disruption and apoptosis: differential regulation of cytochrome c and Smac/DIABLO release. Cancer Res. 63, 1712–1721 (2003).
Jacobson, M. D. et al. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature 361, 365–369 (1993).
He, S. et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137, 1100–1111 (2009).
Liu, Y. et al. Autosis is a Na+, K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc. Natl Acad. Sci. USA 110, 20364–20371 (2013).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Linkermann, A. et al. Synchronized renal tubular cell death involves ferroptosis. Proc. Natl Acad. Sci. USA 111, 16836–16841 (2014).
Ruhrberg, C. & De Palma, M. A double agent in cancer: deciphering macrophage roles in human tumors. Nat. Med. 16, 861–862 (2010).
Guiducci, C., Vicari, A. P., Sangaletti, S., Trinchieri, G. & Colombo, M. P. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 65, 3437–3446 (2005).
Mosser, D. M. & Edwards, J. P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Gao, M., Monian, P., Quadri, N., Ramasamy, R. & Jiang, X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell 59, 298–308 (2015).
Gabizon, A. et al. Cancer nanomedicines: closing the translational gap. Lancet 384, 2175–2176 (2014).
Ma, K., Zhang, D., Cong, Y. & Wiesner, U. Elucidating the mechanism of silica nanoparticle PEGylation processes using fluorescence correlation spectroscopies. Chem. Mater. 28, 1537–1545 (2016).
Roveri, A., Maiorino, M. & Ursini, F. Enzymatic and immunological measurements of soluble and membrane-bound phospholipid-hydroperoxide glutathione peroxidase. Methods Enzymol. 233, 202–212 (1994).
Zeger, S. L., Liang, K. Y. & Albert, P. S. Models for longitudinal data: a generalized estimating equation approach. Biometrics 44, 1049–1060 (1988).
Acknowledgements
This study was funded by grants from the National Institutes of Health (R01GM111350 to M.O.; 1R01CA161280-01A1 to M.B. and U.W.; 1U54 CA199081-01 to M.B. and U.W.; R01GM113013 and R01CA166413 to X.J.; Sloan Kettering Institute Core Grant P30 CA008748CCSG and the Benjamin Friedman Research Fund to M.O.). Peptide synthesis was conducted by the University of Missouri Structural Biology Core.
Author information
Authors and Affiliations
Contributions
Product preparation was performed by K.M., T.Q. and M.Z.T., experimental design by S.E.K., M.O., M.S.B., K.M., L.Z., M.Go., X.J., P.Z., S.M., F.C., T.Q. and U.W., data acquisition by S.E.K., L.Z., K.M., M.Z.T., M.Go., X.J., M.P., F.C., S.M., M.S.B., M.O., I.I., M.C. and M.R., data analysis and interpretation by S.E.K., M.O., M.S.B., K.M., M.P., F.C., P.Z., M.Ga., S.M., L.Z., U.W., I.I., M.C. and M.R., and preparation of the manuscript by S.E.K., M.O., M.S.B., M.Go., P.Z., K.M., S.M., T.Q. and U.W. All authors discussed the results and implications and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have filed an international patent application PCT/US16/34351.
Supplementary information
Supplementary information
Supplementary information (PDF 4037 kb)
Supplementary Movie 1
Supplementary Movie 1 (MOV 1968 kb)
Supplementary Movie 2
Supplementary Movie 2 (MOV 3107 kb)
Rights and permissions
About this article
Cite this article
Kim, S., Zhang, L., Ma, K. et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nature Nanotech 11, 977–985 (2016). https://doi.org/10.1038/nnano.2016.164
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2016.164
This article is cited by
-
Sanguinarine chloride induces ferroptosis by regulating ROS/BACH1/HMOX1 signaling pathway in prostate cancer
Chinese Medicine (2024)
-
Nanomedicine targeting ferroptosis to overcome anticancer therapeutic resistance
Science China Life Sciences (2024)
-
Current and Potential Roles of Ferroptosis in Bladder Cancer
Current Medical Science (2024)
-
Deferoxamine Mitigates Ferroptosis and Inflammation in Hippocampal Neurons After Subarachnoid Hemorrhage by Activating the Nrf2/TXNRD1 Axis
Molecular Neurobiology (2024)
-
Metal organic framework–based variable-size nanoparticles for tumor microenvironment-responsive drug delivery
Drug Delivery and Translational Research (2024)