Abstract
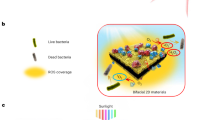
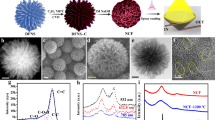
Solar energy is readily available in most climates and can be used for water purification. However, solar disinfection of drinking water mostly relies on ultraviolet light, which represents only 4% of the total solar energy, and this leads to a slow treatment speed. Therefore, the development of new materials that can harvest visible light for water disinfection, and so speed up solar water purification, is highly desirable. Here we show that few-layered vertically aligned MoS2 (FLV-MoS2) films can be used to harvest the whole spectrum of visible light (∼50% of solar energy) and achieve highly efficient water disinfection. The bandgap of MoS2 was increased from 1.3 to 1.55 eV by decreasing the domain size, which allowed the FLV-MoS2 to generate reactive oxygen species (ROS) for bacterial inactivation in the water. The FLV-MoS2 showed a ∼15 times better log inactivation efficiency of the indicator bacteria compared with that of bulk MoS2, and a much faster inactivation of bacteria under both visible light and sunlight illumination compared with the widely used TiO2. Moreover, by using a 5 nm copper film on top of the FLV-MoS2 as a catalyst to facilitate electron–hole pair separation and promote the generation of ROS, the disinfection rate was increased a further sixfold. With our approach, we achieved water disinfection of >99.999% inactivation of bacteria in 20 min with a small amount of material (1.6 mg l–1) under simulated visible light.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Shannon, M. A. et al. Science and technology for water purification in the coming decades. Nature 452, 301–310 (2008).
Schwarzenbach, R. P. et al. The challenge of micropollutants in aquatic systems. Science 313, 1072–1077 (2006).
Liu, C. et al. Conducting nanosponge electroporation for affordable and high-efficiency disinfection of bacteria and viruses in water. Nano Lett. 13, 4288–4293 (2013).
Liu, C. et al. Static electricity powered copper oxide nanowire microbicidal electroporation for water disinfection. Nano Lett. 14, 5603–5608 (2014).
Logan, B. E. & Elimelech, M. Membrane-based processes for sustainable power generation using water. Nature 488, 313–319 (2012).
McGuigan, K. G. et al. Solar water disinfection (SODIS): a review from bench-top to roof-top. J. Hazard. Mater. 235, 29–46 (2012).
Sinha, R. P. & Hader, D. P. UV-induced DNA damage and repair: a review. Photochem. Photobiol. Sci. 1, 225–236 (2002).
Hijnen, W. A. M., Beerendonk, E. F. & Medema, G. J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: a review. Water Res. 40, 3–22 (2006).
Silverman, A. I., Peterson, B. M., Boehm, A. B., McNeill, K. & Nelson, K. L. Sunlight inactivation of human viruses and bacteriophages in coastal waters containing natural photosensitizers. Environ. Sci. Technol. 47, 1870–1878 (2013).
Dong, S. Y. et al. Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: a review. RSC Adv. 5, 14610–14630 (2015).
Dong, S. Y. et al. Designing three-dimensional acicular sheaf shaped BiVO4/reduced graphene oxide composites for efficient sunlight-driven photocatalytic degradation of dye wastewater. Chem. Eng. J. 249, 102–110 (2014).
Dong, S. Y. et al. ZnSnO3 hollow nanospheres/reduced graphene oxide nanocomposites as high-performance photocatalysts for degradation of metronidazole. Appl. Catal. B 144, 386–393 (2014).
Chong, M. N., Jin, B., Chow, C. W. K. & Saint, C. Recent developments in photocatalytic water treatment technology: a review. Water Res. 44, 2997–3027 (2010).
Malato, S., Fernandez-Ibanez, P., Maldonado, M. I., Blanco, J. & Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: recent overview and trends. Catal. Today 147, 1–59 (2009).
Wardman, P. Reduction potentials of one-electron couples involving free-radicals in aqueous-solution. J. Phys. Chem. Ref. Data 18, 1637–1755 (1989).
Wood, P. M. The potential diagram for oxygen at pH-7. Biochem. J. 253, 287–289 (1988).
Lawless, D., Serpone, N. & Meisel, D. Role of OH• radicals and trapped holes in photocatalysis—a pulse radiolysis study. J. Phys. Chem. 95, 5166–5170 (1991).
Liao, H. D. & Reitberger, T. Generation of free OHaq radicals by black light illumination of Degussa (Evonik) P25 TiO2 aqueous suspensions. Catalysts 3, 418–443 (2013).
Chen, X. & Mao, S. S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 107, 2891–2959 (2007).
Li, Q., Xie, R. C., Ll, Y. W., Mintz, E. A. & Shang, J. K. Enhanced visible-light-induced photocatalytic disinfection of E. coli by carbon-sensitized nitrogen-doped titanium oxide. Environ. Sci. Technol. 41, 5050–5056 (2007).
Cong, Y., Zhang, J. L., Chen, F., Anpo, M. & He, D. N. Preparation, photocatalytic activity, and mechanism of nano-TiO2 co-doped with nitrogen and iron (III). J. Phys. Chem. C 111, 10618–10623 (2007).
Asahi, R., Morikawa, T., Ohwaki, T., Aoki, K. & Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293, 269–271 (2001).
Choi, J., Park, H. & Hoffmann, M. R. Effects of single metal-ion doping on the visible-light photoreactivity of TiO2 . J. Phys. Chem. C 114, 783–792 (2010).
Yu, J. C. et al. Efficient visible-light-induced photocatalytic disinfection on sulfur-doped nanocrystalline titania. Environ. Sci. Technol. 39, 1175–1179 (2005).
Hayden, S. C., Allam, N. K. & El-Sayed, M. A. TiO2 nanotube/CdS hybrid electrodes: extraordinary enhancement in the inactivation of Escherichia coli. J. Am. Chem. Soc. 132, 14406–14408 (2010).
Chen, C. et al. Synthesis of visible-light responsive graphene oxide/TiO2 composites with p/n heterojunction. ACS Nano 4, 6425–6432 (2010).
Yu, J. G., Dai, G. P. & Huang, B. B. Fabrication and characterization of visible-light-driven plasmonic photocatalyst Ag/AgCl/TiO2 nanotube arrays. J. Phys. Chem. C 113, 16394–16401 (2009).
Mor, G. K., Varghese, O. K., Paulose, M., Shankar, K. & Grimes, C. A. A review on highly ordered, vertically oriented TiO2 nanotube arrays: fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. Cells 90, 2011–2075 (2006).
Tao, J. G., Luttrell, T. & Batzill, M. A two-dimensional phase of TiO2 with a reduced bandgap. Nature Chem. 3, 296–300 (2011).
Dette, C. et al. TiO2 anatase with a bandgap in the visible region. Nano Lett. 14, 6533–6538 (2014).
Huang, J. H., Ho, W. K. & Wang, X. C. Metal-free disinfection effects induced by graphitic carbon nitride polymers under visible light illumination. Chem. Commun. 50, 4338–4340 (2014).
Wang, X. C. et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nature Mater. 8, 76–80 (2009).
Xia, D. H. et al. Red phosphorus: an Earth-abundant elemental photocatalyst for ‘green’ bacterial inactivation under visible light. Environ. Sci. Technol. 49, 6264–6273 (2015).
Gao, P., Liu, J. C., Sun, D. D. & Ng, W. Graphene oxide–CdS composite with high photocatalytic degradation and disinfection activities under visible light irradiation. J. Hazard. Mater. 250, 412–420 (2013).
Gao, P., Ng, K. & Sun, D. D. Sulfonated graphene oxide–ZnO–Ag photocatalyst for fast photodegradation and disinfection under visible light. J. Hazard. Mater. 262, 826–835 (2013).
Wang, W. J. et al. Visible-light-driven photocatalytic inactivation of E. coli K-12 by bismuth vanadate nanotubes: bactericidal performance and mechanism. Environ. Sci. Technol. 46, 4599–4606 (2012).
Wang, W. J., Yu, J. C., Xia, D. H., Wong, P. K. & Li, Y. C. Graphene and g-C3N4 nanosheets co-wrapped elemental α-sulfur as a novel metal-free heterojunction photocatalyst for bacterial inactivation under visible-light. Environ. Sci. Technol. 47, 8724–8732 (2013).
Radisavljevic, B., Radenovic, A., Brivio, J., Giacometti, V. & Kis, A. Single-layer MoS2 transistors. Nature Nanotechnol. 6, 147–150 (2011).
Wang, Q. H., Kalantar-Zadeh, K., Kis, A., Coleman, J. N. & Strano, M. S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nature Nanotechnol. 7, 699–712 (2012).
Jaramillo, T. F. et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 (2007).
Kong, D. S. et al. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 13, 1341–1347 (2013).
Wang, H. T. et al. Electrochemical tuning of vertically aligned MoS2 nanofilms and its application in improving hydrogen evolution reaction. Proc. Natl Acad. Sci. USA 110, 19701–19706 (2013).
Mak, K. F., Lee, C., Hone, J., Shan, J. & Heinz, T. F. Atomically thin MoS2: a new direct-gap semiconductor. Phys. Rev. Lett. 105, 136805 (2010).
Tong, H. et al. Nano-photocatalytic materials: possibilities and challenges. Adv. Mater. 24, 229–251 (2012).
Sakthivel, S. et al. Enhancement of photocatalytic activity by metal deposition: characterisation and photonic efficiency of Pt, Au and Pd deposited on TiO2 catalyst. Water Res. 38, 3001–3008 (2004).
Li, H. X. et al. Mesoporous Au/TiO2 nanocomposites with enhanced photocatalytic activity. J. Am. Chem. Soc. 129, 4538–4539 (2007).
Subramanian, V., Wolf, E. E. & Kamat, P. V. Catalysis with TiO2/gold nanocomposites. Effect of metal particle size on the Fermi level equilibration. J. Am. Chem. Soc. 126, 4943–4950 (2004).
Bai, H. W., Liu, Z. Y. & Sun, D. D. Hierarchical ZnO/Cu ‘corn-like’ materials with high photodegradation and antibacterial capability under visible light. Phys. Chem. Chem. Phys. 13, 6205–6210 (2011).
Acknowledgements
We acknowledge the Stanford facilities, Stanford Nanocharacterization Laboratory and Soft & Hybrid Materials, for characterization. Y.C. acknowledges support from the US Department of Energy, Basic Energy Sciences, Materials Sciences and Engineering Division, under contract DE-AC02-76SF00515. We thank G. M. Stewart for his help with the schematic drawing.
Author information
Authors and Affiliations
Contributions
C.L. and Y.C. developed the concept. C.L. synthesized the samples and conducted the disinfection measurement and material characterizations. D.K. and H.W. helped with the material synthesis. P.-C.H. and S.W. helped with the optical measurement. H.Y. helped with the Kelvin probe measurement. H.-W.L. did the TEM characterization. D.K. helped with the Raman spectroscopy measurement. Y.L. helped with catalyst measurements. P.A.M. helped with estimation of the real-sunlight spectrum. K.M.P. helped with HPLC measurement. C.L., A.B.B. and Y.C. analysed the data and co-wrote the paper. K.Y. and D.L. provided important experimental insights. All the authors discussed the whole paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1638 kb)
Rights and permissions
About this article
Cite this article
Liu, C., Kong, D., Hsu, PC. et al. Rapid water disinfection using vertically aligned MoS2 nanofilms and visible light. Nature Nanotech 11, 1098–1104 (2016). https://doi.org/10.1038/nnano.2016.138
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2016.138
This article is cited by
-
A bibliometric analysis of molybdenum-based nanomaterials in the biomedical field
Tungsten (2024)
-
Tooth whitening and caries prevention toothbrush based on PTFE electret
Journal of Materials Science (2024)
-
Exfoliation, functionalization and antibacterial activity of transition metal dichalcogenides
Tungsten (2024)
-
The current status of stimuli-responsive nanotechnologies on orthopedic titanium implant surfaces
Journal of Nanobiotechnology (2023)
-
Solar-driven efficient heterogeneous subminute water disinfection nanosystem assembled with fingerprint MoS2
Nature Water (2023)