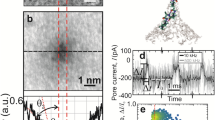
Abstract
The primary structure of a protein consists of a sequence of amino acids and is a key factor in determining how a protein folds and functions. However, conventional methods for sequencing proteins, such as mass spectrometry and Edman degradation, suffer from short reads and lack sensitivity, so alternative approaches are sought. Here, we show that a subnanometre-diameter pore, sputtered through a thin silicon nitride membrane, can be used to detect the primary structure of a denatured protein molecule. When a denatured protein immersed in electrolyte is driven through the pore by an electric field, measurements of a blockade in the current reveal nearly regular fluctuations, the number of which coincides with the number of residues in the protein. Furthermore, the amplitudes of the fluctuations are highly correlated with the volumes that are occluded by quadromers (four residues) in the primary structure. Each fluctuation, therefore, represents a read of a quadromer. Scrutiny of the fluctuations reveals that the subnanometre pore is sensitive enough to read the occluded volume that is related to post-translational modifications of a single residue, measuring volume differences of ∼0.07 nm3, but it is not sensitive enough to discriminate between the volumes of all twenty amino acids.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wilhem, M. et al. Mass-spectrometry-based draft of the human proteome. Nature 509, 582–587 (2014).
Altelaar, A. F. M., Munoz, J. & Heck, A. J. R. Next-generation proteomics: towards an integrative view of proteome dynamics. Nat. Rev. Genet. 14, 35–48 (2013).
Chandramouli, K. & Qian, P.-Y. Proteomics: challenges, techniques and possibilities to overcome biological sample complexity. Human Genom. Proteom. 2009, 239204 (2009).
Ohshiro, T. et al. Detection of post-translational modifications in single peptides using electron tunneling currents. Nature Nanotech. 9, 835–840 (2014).
Movileanu, L., Howorka, S., Braha, O. & Bayley, H. Detecting protein analytes that modulate transmembrane movement of a polymer chain within a single protein pore. Nature Biotechnol. 18, 1091–1095 (2000).
Mohammad, M. M., Prakash, S., Matouschek, A. & Movileanu, L. Controlling a single protein in a nanopore through electrostatic traps. J. Am. Chem. Soc. 130, 4081–4088 (2008).
Talaga, D. S. & Li, J. Single-molecule protein unfolding in solid-state nanopores. J. Am. Chem. Soc. 131, 9287–9297 (2009).
Wei, R., Gatterdam, V., Wieneke, R., Tampe, R. & Rant, U. Stochastic sensing of proteins with receptor-modified solid-state nanopores. Nature Nanotech. 7, 257–263 (2012).
Freedman, K. J. et al. Chemical, thermal, and electric field-induced unfolding of single protein molecules studied using nanopores. Anal. Chem. 83, 5137–5144 (2011).
Fologea, D., Ledden, B., McNabb, D. S. & Li, J. Electrical characterization of protein molecules by a solid-state nanopore. Appl. Phys. Lett. 91, 053901 (2007).
Cressiot, B. et al. Protein transport through a narrow solid-state nanopore at high voltage: experiments and theory. ACS Nano 6, 6236–6243 (2012).
Nelson, E. M., Kurz, V., Shim, J., Timp, W. & Timp, G. Using a nanopore for single molecule detection and single cell transfection. Analyst 137, 3020–3027 (2012).
Nivala, J., Marks, D. B. & Akeson, M. Unfoldase-mediated protein translocation through an α-hemolysin nanopore. Nature Biotechnol. 31, 247–250 (2013).
Rodriguez-Larrea, D. & Bayley, H. Multistep protein unfolding during nanopore translocation. Nature Nanotech. 8, 288–295 (2013).
Rosen, C. B., Rodriguez-Larrea, D. & Bayley, H. Single-molecule site-specific detection of protein phosphorylation with a nanopore. Nature Biotechnol. 32, 179–181 (2014).
Merstorf, C. et al. Wild type, mutant protein unfolding and phase transition detected by single-nanopore recording. ACS Chem. Biol. 7, 652–658 (2012).
Nivala, J., Mulroney, L., Li, G., Schreiber, J. & Akeson, M. Discrimination among protein variants using an unfoldase-coupled nanopore. ACS Nano 8, 12365–12375 (2014).
Laszlo, A. H. et al. Decoding long nanopore sequencing reads of natural DNA. Nature Biotechnol. 32, 829–833 (2014).
Jain, M. et al. Improved data analysis for the MinION nanopore sequencer. Nature Methods 12, 351–356 (2015).
Timp, W. et al. Think small: nanopores for sensing and synthesis. IEEE Access 2, 1396–1408 (2014).
Laver, T. et al. Assessing the performance of the Oxford Nanopore Technologies MinION. Biomol. Detect. Quant. 3, 1–8 (2015).
Li, J., Fologea, D., Rollings, R. & Ledden, B. Characterization of protein unfolding with solid-state nanopores. Protein Pept. Lett. 21, 256–265 (2014).
Sigalov, G., Comer, J., Timp, G. & Aksimentiev, A. Detection of DNA sequences using an alternating electric field in a nanopore capacitor. Nano Lett. 8, 56–63 (2008).
Ho, C. et al. Electrolytic transport through a synthetic nanometer-diameter pore. Proc. Natl Acad. Sci. USA 102, 10445–10450 (2005).
Ohtaki, H. & Radnai, T. Structure and dynamics of hydrated ions. Chem. Rev. 93, 1157–1204 (1993).
Reynolds, J. A. & Tanford, C. Binding of dodecyl sulfate to proteins at high binding ratios. possible implications for the state of proteins in biological membranes. Proc. Natl Acad. Sci. USA 66, 1002–1003 (1970).
Ibel, K. et al. Protein-decorated micelle structure of sodium dodecyl-sulfate-protein complexes as determined by neutron scattering. Eur. J. Biochem. 190, 311–318 (1990).
Samso, M., Daban, J.-R., Hansen, S. & Jones, G. R. Evidence for sodium dodecyl sulfate/protein complexes adopting a necklace structure. Eur. J. Biochem. 232, 818–824 (1995).
Mattice, W. L., Riser, J. M. & Clark, D. S. Conformational properties of the complexes formed by proteins and sodium dodecyl sulfate. Biochemistry 15, 4264–4272 (1976).
Lundahl, P., Greijer, E., Sandberg, M., Cardell, S. & Eriksson, K. O. A model for ionic and hydrophobic interactions and hydrogen-bonding in sodium dodecyl sulfate-protein complexes. Biochim. Biophys. Acta 873, 20–26 (1986).
Gudiksen, K. L., Gitlin, I., Moustakas, D. T. & Whitesides, G. M. Increasing the net charge and decreasing the hydrophobicity of bovine carbonic anhydrase decreases the rate of denaturation with sodium dodecyl sulfate. Biophys. J. 91, 298–310 (2006).
Westerhuis, W. H. J., Sturgis, J. N. & Niederman, R. A. Reevaluation of the electrophoretic migration behavior of soluble globular proteins in the native and detergent-denatured states in polyacrylamide gels. Anal. Biochem. 284, 143–152 (2000).
Nakayma, J.-I., Rice, J. C., Strahl, B. D., Allis, C. D. & Grewal, S. I. S. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292, 110–113 (2001).
Wang, Z. et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nature Genet. 40, 897–903 (2008).
Shortle, D. & Ackerman, M. S. Persistence of native-like topology in a denatured protein in 8 M urea. Science 293, 487–489 (2001).
Qin, Z. P., Zhe, J. A. & Wang, G. X. Effects of particle's off-axis position, shape, orientation and entry position on resistance changes of micro-Coulter counting devices. Meas. Sci. Technol. 22, 045804 (2011).
Niedzwiecki, D. J., Grazul, J. & Movileanu, L. Single-molecule observation of protein adsorption onto an inorganic surface. J. Am. Chem. Soc. 132, 10816–10822 (2010).
Székely, G. J. & Rizzo, M. L. A new test for multivariate normality. J. Multivar. Anal. 93, 58–80 (2005).
Nelson, E. M., Li, H. & Timp, G. Direct, concurrent measurements of the forces and currents affecting DNA in a nanopore with comparable topography. ACS Nano 8, 5484–5493 (2014).
Perkins, S. J. Protein volumes and hydration effects. Eur. J. Biochem. 157, 169–180 (1986).
Timp, W., Comer, J. & Aksimentiev, A. DNA base-calling from a nanopore using a Viterbi algorithm. Biophys. J. 102, L37–L39 (2012).
Friedman, J., Hastie, T. & Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 33, 1–22 (2010).
Kolmogorov, M., Kennedy, E., Dong, Z., Timp, G. & Pevzner, P. Single-molecule protein identification by subnanopore sensors. Preprint at https://arxiv.org/abs/1604.02270v1 (2016).
Barthel, J. Dr. Probe-High-resolution (S)TEM image simulation software. http://www.er-c.org/barthel/drprobe, version 1.6 (2015).
Cowley, J. M. & Moodie, A. F. The scattering of electrons by atoms and crystals. I. a new theoretical approach. Acta. Cryst. 10, 609–619 (1957).
Weickenmeier, A. & Kohl, H. Computation of absorptive form factors for high-energy electron diffraction. Acta Cryst. A 47, 590–597 (1991).
Raillon, C., Granjon, P., Graf, M., Steinbocka, L. J. & Radenovic, A. Fast and automatic processing of multi-level events in nanopore translocation experiments. Nanoscale 4, 4916–4924 (2012).
Kurz, V., Nelson, E. M., Shim, J. & Timp, G. Direct visualization of single-molecule translocations through synthetic nanopores comparable in size to a molecule. ACS Nano 7, 4057–4069 (2013).
Grahame, D. C. The electrical double layer and the theory of electrocapillarity. Chem. Rev. 41, 441–501 (1947).
Acknowledgements
This work was partially supported by a grant from the National Science Foundation (DBI 1256052), the Keough–Hesburgh Professorship and the Walther Cancer Foundation. We gratefully acknowledge conversations with J. Barthel and critical readings of the manuscript by W. Timp and T. Tanaka.
Author information
Authors and Affiliations
Contributions
G.T. conceived the experiments. E.K. and Z.D. designed the experiments in consultation with G.T. E.K., Z.D. and C.T. performed the experiments and analysed the data. E.K. developed the algorithms used to analyse the current blockade data, in particular. G.T., E.K. and Z.D. co-wrote the manuscript, but all of the authors discussed the results and commented on it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2758 kb)
Rights and permissions
About this article
Cite this article
Kennedy, E., Dong, Z., Tennant, C. et al. Reading the primary structure of a protein with 0.07 nm3 resolution using a subnanometre-diameter pore. Nature Nanotech 11, 968–976 (2016). https://doi.org/10.1038/nnano.2016.120
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2016.120
This article is cited by
-
Peptide sequencing based on host–guest interaction-assisted nanopore sensing
Nature Methods (2024)
-
Molecular sensitised probe for amino acid recognition within peptide sequences
Nature Communications (2023)
-
Unidirectional single-file transport of full-length proteins through a nanopore
Nature Biotechnology (2023)
-
Comprehensive structural assignment of glycosaminoglycan oligo- and polysaccharides by protein nanopore
Nature Communications (2022)
-
Calling the amino acid sequence of a protein/peptide from the nanospectrum produced by a sub-nanometer diameter pore
Scientific Reports (2022)