Abstract
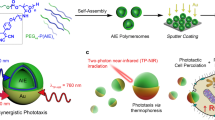
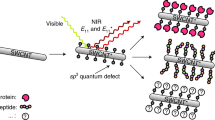
Receptor-mediated signal transduction modulates complex cellular behaviours such as cell growth, migration and differentiation. Although photoactivatable proteins have emerged as a powerful tool for controlling molecular interactions and signalling cascades at precise times and spaces using light, many of these light-sensitive proteins are activated by ultraviolent or visible light, which has limited tissue penetration. Here, we report a single-walled carbon nanotube (SWCNT)-assisted approach that enables near-infrared light-triggered activation of transforming growth factor β (TGF-β) signal transduction, an important signalling pathway in embryonic development and cancer progression. The protein complex of TGF-β and its latency-associated peptide is conjugated onto SWCNTs, where TGF-β is inactive. Upon near-infrared irradiation, TGF-β is released through the photothermal effect of SWCNTs and becomes active. The released TGF-β activates downstream signal transduction in live cells and modulates cellular behaviours. Furthermore, preliminary studies show that the method can be used to mediate TGF-β signalling in living mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Feng, X.-H. & Derynck, R. Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 21, 659–693 (2005).
Annes, J. P., Munger, J. S. & Rifkin, D. B. Making sense of latent TGFβ activation. J. Cell Sci. 116, 217–224 (2003).
Munger, J. S. et al. The integrin αVβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96, 319–328 (1999).
Shi, M. et al. Latent TGF-β structure and activation. Nature 474, 343–349 (2011).
Wu, M. Y. & Hill, C. S. TGF-β superfamily in embryonic development and homeostasis. Dev. Cell 16, 329–343 (2009).
Massagué, J. TGFβ in cancer. Cell 134, 215–230 (2008).
Gorostiza, P. & Isacoff, E. Y. Optical switches for remote and noninvasive control of cell signaling. Science 322, 395–399 (2008).
Toettcher, J. E., Voigt, C. A., Weiner, O. D. & Lim, W. A. The promise of optogenetics in cell biology: interrogating molecular circuits in space and time. Nature Methods 8, 35–38 (2011).
Ellis-Davies, G. C. R. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nature Methods 4, 619–628 (2007).
Fortin, D. L. et al. Photochemical control of endogenous ion channels and cellular excitability. Nature Methods 5, 331–338 (2008).
Airan, R. D., Thompson, K. R., Fenno, L. E., Bernstein, H. & Deisseroth, K. Temporally precise in vivo control of intracellular signalling. Nature 458, 1025–1029 (2009).
Wu, Y. I. et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461, 104–108 (2009).
Levskaya, A., Weiner, O. D., Lim, W. A. & Voigt, C. A. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature 461, 997–1001 (2009).
O'Connell, M. J. et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 297, 593–596 (2002).
Shi Kam, N. W., O'Connell, M., Wisdom, J. A. & Dai, H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc. Natl Acad. Sci. USA 102, 11600–11605 (2005).
Chakravarty, P. et al. Thermal ablation of tumor cells with antibody-functionalized single-walled carbon nanotubes. Proc. Natl Acad. Sci. USA 105, 8697–8702 (2008).
Joshi, A. et al. Nanotube-assisted protein deactivation. Nature Nanotech. 3, 41–45 (2008).
Liu, Z., Tabakman, S. M., Chen, Z. & Dai, H. Preparation of carbon nanotube bioconjugates for biomedical applications. Nature Protoc. 4, 1372–1382 (2009).
Rastogi, R. et al. Comparative study of carbon nanotube dispersion using surfactants. J. Colloid Interface Sci. 328, 421–428 (2008).
Wittig, I., Braun, H-P. & Schägger, H. Blue native PAGE. Nature Protoc. 1, 418–428 (2006).
Dennler, S. et al. Direct binding of Smad3 and Smad4 to critical TGFβ-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 17, 3091–3100 (1998).
Riedinger, A. et al. Subnanometer local temperature probing and remotely controlled drug release based on azo-functionalized iron oxide nanoparticles. Nano Lett. 13, 2399–2406 (2013).
Murakami, T. et al. Photodynamic and photothermal effects of semiconducting and metallic-enriched single-walled carbon nanotubes. J. Am. Chem. Soc. 134, 17862–17865 (2012).
Greffrath, W. et al. Inward currents in primary nociceptive neurons of the rat and pain sensations in humans elicited by infrared diode laser pulses. Pain 99, 145–155 (2002).
Lim, Y. T. et al. Selection of quantum dot wavelengths for biomedical assays and imaging. Mol. Imaging 2, 50–64 (2003).
Welsher, K. et al. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nature Nanotech. 4, 773–780 (2009).
Idris, N. M. et al. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nature Med. 18, 1580–1585 (2012).
Kam, N. W. S., Liu, Z. & Dai, H. Carbon nanotubes as intracellular transporters for proteins and DNA: an investigation of the uptake mechanism and pathway. Angew. Chem. Int. Ed. 45, 577–581 (2006).
Xu, J., Lamouille, S. & Derynck, R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 19, 156–172 (2009).
Laiho, M., DeCaprio, J. A., Ludlow, J. W., Livingston, D. M. & Massagué, J. Growth inhibition by TGF-β linked to suppression of retinoblastoma protein phosphorylation. Cell 62, 175–185 (1990).
Li, J. M., Hu, P. P., Shen, X., Yu, Y. & Wang, X. F. E2F4-RB and E2F4-p107 complexes suppress gene expression by transforming growth factor β through E2F binding sites. Proc. Natl Acad. Sci. USA 94, 4948–4953 (1997).
Hirsch, L. R. et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl Acad. Sci. USA 100, 13549–13554 (2003).
Giljohann, D. A. et al. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. 49, 3280–3294 (2010).
Yang, K. et al. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 10, 3318–3323 (2010).
Robinson, J. T. et al. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 133, 6825–6831 (2011).
Rickgauer, J. P. & Tank, D. W. Two-photon excitation of channelrhodopsin-2 at saturation. Proc. Natl Acad. Sci. USA 106, 15025–15030 (2009).
Izquierdo-Serra, M. et al. Two-photon neuronal and astrocytic stimulation with azobenzene-based photoswitches. J. Am. Chem. Soc. 136, 8693–8701 (2014).
Papagiakoumou, E. et al. Scanless two-photon excitation of channelrhodopsin-2. Nature Methods 7, 848–854 (2010).
Prakash, R. et al. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nature Methods 9, 1171–1179 (2012).
Xu, C. & Webb, W. W. Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm. J. Opt. Soc. Am. B 13, 481–491 (1996).
Acknowledgements
This work was supported by the National Basic Research Program of China (973 program; 2012CB917303) and the National Natural Science Foundation of China (nos. 91313301, 21425204 and 91127034).
Author information
Authors and Affiliations
Contributions
X.C. conceived the original idea and designed the experiments. L.L. and L.L., assisted by B.Z., R.X., W.L., H.L. and Y.L., performed experiments. M.S., Y.C. and T.A.S. contributed materials. L.L., L.L. and X.C. analysed the data and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 766 kb)
Rights and permissions
About this article
Cite this article
Lin, L., Liu, L., Zhao, B. et al. Carbon nanotube-assisted optical activation of TGF-β signalling by near-infrared light. Nature Nanotech 10, 465–471 (2015). https://doi.org/10.1038/nnano.2015.28
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2015.28
This article is cited by
-
Copper sulfide nanoparticles as a photothermal switch for TRPV1 signaling to attenuate atherosclerosis
Nature Communications (2018)