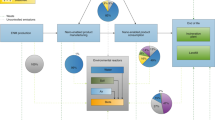
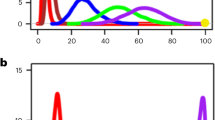
Abstract
The need to assess the human and environmental risks of nanoscale materials has prompted the development of new metrological tools for their detection, quantification and characterization. Some of these methods have tremendous potential for use in various scenarios of nanotoxicology. However, in some cases, the limited dialogue between environmental scientists and human toxicologists has hampered the full exploitation of these resources. Here we review recent progress in the development of methods for nanomaterial analysis and discuss the use of these methods in environmental and human toxicology. We highlight the opportunities for collaboration between these two research areas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Nowack, B. et al. Potential scenarios for nanomaterial release and subsequent alteration in the environment. Environ. Toxicol. Chem. 31, 50–59 (2012).
Von der Kammer, F. et al. Analysis of engineered nanomaterials in complex matrices (environment and biota): General considerations and conceptual case studies. Environ. Toxicol. Chem. 31, 32–49 (2012).
Montaño, M. D., Lowry, G. V., von der Kammer, F., Blue, J. & Ranville, J. F. Current status and future direction for examining engineered nanoparticles in natural systems. Environ. Chem. 11, 351–366 (2014). We recommend this review for a synopsis of available techniques for the detection and characterization of engineered nanoparticles in complex samples.
Meesters, J. A., Veltman, K., Hendriks, A. J. & van de Meent, D. Environmental exposure assessment of engineered nanoparticles: why REACH needs adjustment. Integr. Environ. Assess. Manag. 9, e15–e26 (2013). A comprehensive discussion of how specific physicochemical properties of nanomaterials should be addressed to achieve sufficient applicability of the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) method to their risk assessment.
Godwin, H. et al. Nanomaterial categorization for assessing risk potential to facilitate regulatory decision-making. ACS Nano 9, 3409–3417 (2015).
Lespes, G. & Gigault, J. Hyphenated analytical techniques for multidimensional characterisation of submicron particles: a review. Anal. Chim. Acta 692, 26–41 (2011).
Tiede, K. et al. Application of hydrodynamic chromatography-ICP-MS to investigate the fate of silver nanoparticles in activated sludge. J. Anal. At. Spectrom. 25, 1149–1154 (2010).
Tiede, K. et al. A robust size-characterisation methodology for studying nanoparticle behaviour in 'real' environmental samples, using hydrodynamic chromatography coupled to ICP-MS. J. Anal. At. Spectrom. 24, 964–972 (2009).
Baalousha, M., Stolpe, B. & Lead, J. Flow field-flow fractionation for the analysis and characterization of natural colloids and manufactured nanoparticles in environmental systems: a critical review. J. Chromatogr. 1218, 4078–4103 (2011).
Kammer, F. v. d., Legros, S., Hofmann, T., Larsen, E. H. & Loeschner, K. Separation and characterization of nanoparticles in complex food and environmental samples by field-flow fractionation. Trends Anal. Chem. 30, 425–436 (2011).
Meisterjahn, B., Neubauer, E., von der Kammer, F., Hennecke, D. & Hofmann, T. Asymmetrical flow-field-flow fractionation coupled with inductively coupled plasma mass spectrometry for the analysis of gold nanoparticles in the presence of natural nanoparticles. J. Chromatogr. 1372, 204–211 (2014).
Montaño, M., Badiei, H., Bazargan, S. & Ranville, J. Improvements in the detection and characterization of engineered nanoparticles using spICP-MS with microsecond dwell times. Env. Sci. Nano 1, 338–346 (2014).
Borovinskaya, O., Gschwind, S., Hattendorf, B., Tanner, M. & Gu¨nther, D. Simultaneous mass quantification of nanoparticles of different composition in a mixture by microdroplet generator-ICPTOFMS. Anal. Chem. 86, 8142–8148 (2014).
Cornelis, G. & Hassellov, M. A signal deconvolution method to discriminate smaller nanoparticles in single particle ICP-MS. J. Anal. At. Spectrom. 29, 134–144 (2014).
Hineman, A. & Stephan, C. Effect of dwell time on single particle inductively coupled plasma mass spectrometry data acquisition quality. J. Anal. At. Spectrom. 29, 1252–1257 (2014).
Pergantis, S. A., Jones-Lepp, T. L. & Heithmar, E. M. Hydrodynamic chromatography online with single particle-inductively coupled plasma mass spectrometry for ultratrace detection of metal-containing nanoparticles. Anal. Chem. 84, 6454–6462 (2012).
Loeschner, K. et al. Detection and characterization of silver nanoparticles in chicken meat by asymmetric flow field flow fractionation with detection by conventional or single particle ICP-MS. Anal. Bioanal. Chem. 405, 8185–8195 (2013).
Lee, S. et al. Nanoparticle size detection limits by single particle ICP-MS for 40 elements. Environ. Sci. Technol. 48, 10291–10300 (2014).
Singh, G., Stephan, C., Westerhoff, P., Carlander, D. & Duncan, T. V. Measurement methods to detect, characterize, and quantify engineered nanomaterials in foods. Comp. Rev. Food Sci. Food Safety 13, 693–704 (2014).
Love, S. A., Maurer-Jones, M. A., Thompson, J. W., Lin, Y.-S. & Haynes, C. L. Assessing nanoparticle toxicity. Annu. Rev. Anal. Chem. 5, 181–205 (2012).
Jones, C. F. & Grainger, D. W. 'In vitro' assessments of nanomaterial toxicity. Adv. Drug Del. Rev. 61, 438–456 (2009).
Murdock, R. C., Braydich-Stolle, L., Schrand, A. M., Schlager, J. J. & Hussain, S. M. Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol. Sci. 101, 239–253 (2008).
Tejamaya, M., Ro¨mer, I., Merrifield, R. C. & Lead, J. R. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ. Sci. Technol. 46, 7011–7017 (2012).
Malysheva, A. et al. Sorption of silver nanoparticles to laboratory plastic during (eco)toxicological testing. Nanotoxicology DOI:10.3109/17435390.2015.1084059 (in the press).
Schultz, A. G. et al. Aquatic toxicity of manufactured nanomaterials: challenges and recommendations for future toxicity testing. Environ. Chem. 11, 207–226 (2014).
Cupi, D., Hartmann, N. B. & Baun, A. The influence of natural organic matter and aging on suspension stability in guideline toxicity testing of silver, zinc oxide, and titanium dioxide nanoparticles with Daphnia magna. Environ. Toxicol. Chem. 34, 497–506 (2015).
Slaveykova, V. I., Guignard, C., Eybe, T., Migeon, H.-N. & Hoffmann, L. Dynamic NanoSIMS ion imaging of unicellular freshwater algae exposed to copper. Anal. Bioanal. Chem. 393, 583–589 (2009).
Audinot, J. N. et al. Identification and localization of nanoparticles in tissues by mass spectrometry. Surf. Interface Anal. 45, 230–233 (2013).
Tsang, C.-N., Ho, K.-S., Sun, H. & Chan, W.-T. Tracking bismuth antiulcer drug uptake in single helicobacter pylori cells. J. Am. Chem. Soc. 133, 7355–7357 (2011).
Ho, K.-S. & Chan, W.-T. Time-resolved ICP-MS measurement for single-cell analysis and on-line cytometry. J. Anal. At. Spectrom. 25, 1114–1122 (2010).
Zheng, L.-N. et al. Determination of quantum dots in single cells by inductively coupled plasma mass spectrometry. Talanta 116, 782–787 (2013).
Groombridge, A. S. et al. High sensitive elemental analysis of single yeast cells (Saccharomyces cerevisiae) by time-resolved inductively-coupled plasma mass spectrometry using a high efficiency cell introduction system. Anal. Sci. 29, 597–603 (2013).
Vranic, S. et al. Deciphering the mechanisms of cellular uptake of engineered nanoparticles by accurate evaluation of internalization using imaging flow cytometry. Part. Fibre Toxicol. 10, 2 (2013).
Bendall, S. C. et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696 (2011).
Bandura, D. R. et al. Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal. Chem. 81, 6813–6822 (2009).
Seekell, K., Price, H., Marinakos, S. & Wax, A. Optimization of immunolabeled plasmonic nanoparticles for cell surface receptor analysis. Methods 56, 310–316 (2012).
Badireddy, A. R., Wiesner, M. R. & Liu, J. Detection, characterization, and abundance of engineered nanoparticles in complex waters by hyperspectral imagery with enhanced darkfield microscopy. Environ. Sci. Technol. 46, 10081–10088 (2012).
Fairbairn, N., Christofidou, A., Kanaras, A. G., Newman, T. A. & Muskens, O. L. Hyperspectral darkfield microscopy of single hollow gold nanoparticles for biomedical applications. Phys. Chem. Chem. Phys. 15, 4163–4168 (2013).
Mortimer, M. et al. Potential of hyperspectral imaging microscopy for semi-quantitative analysis of nanoparticle uptake by protozoa. Environ. Sci. Technol. 48, 8760–8767 (2014).
Lombi, E., Scheckel, K. G. & Kempson, I. M. In situ analysis of metal(loid)s in plants: State of the art and artefacts. Environ. Exp. Bot. 72, 3–17 (2011).
McRae, R., Bagchi, P., Sumalekshmy, S. & Fahrni, C. J. In situ imaging of metals in cells and tissues. Chem. Rev. 109, 4780–4827 (2009).
Hernandez-Viezcas, J. A. et al. In situ synchrotron X-ray fluorescence mapping and speciation of CeO2 and ZnO nanoparticles in soil cultivated soybean (Glycine max). ACS Nano 7, 1415–1423 (2013).
Duke, E. M. et al. Imaging endosomes and autophagosomes in whole mammalian cells using correlative cryo-fluorescence and cryo-soft X-ray microscopy (cryo-CLXM). Ultramicroscopy 143, 77–87 (2014).
Parkinson, D. et al. Nanoimaging (eds Sousa, A. A. & Kruhlak, M. J.) Ch. 25, 457–481 (Methods in Molecular Biology Vol. 950, Springer, 2013).
Gräfe, M., Donner, E., Collins, R. N. & Lombi, E. Speciation of metal(loid)s in environmental samples by X-ray absorption spectroscopy: A critical review. Anal. Chim. Acta 822, 1–22 (2014).
Ma, H., Williams, P. L. & Diamond, S. A. Ecotoxicity of manufactured ZnO nanoparticles — A review. Environ. Pollut. 172, 76–85 (2013).
Osmond-McLeod, M. J. et al. Dermal absorption and short-term biological impact in hairless mice from sunscreens containing zinc oxide nano-or larger particles. Nanotoxicology 8, 72–84 (2013).
Lombi, E. et al. Fate of zinc oxide nanoparticles during anaerobic digestion of wastewater and post-treatment processing of sewage sludge. Environ. Sci. Technol. 46, 9089–9096 (2012).
Tenzer, S. et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nature Nanotech. 8, 772–781 (2013).
Monopoli, M. P., Åberg, C., Salvati, A. & Dawson, K. A. Biomolecular coronas provide the biological identity of nanosized materials. Nature Nanotech. 7, 779–786 (2012).
Ju, S. & Yeo, W.-S. Quantification of proteins on gold nanoparticles by combining MALDI-TOF MS and proteolysis. Nanotechnology 23, 135701 (2012).
Walkey C. D. et al. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 134, 2139–2147 (2012).
Suman, T., Rajasree, S. R. & Kirubagaran, R. Evaluation of zinc oxide nanoparticles toxicity on marine algae Chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotoxicol. Environ. Saf. 113, 23–30 (2015).
Kumar, A., Pandey, A. K., Singh, S. S., Shanker, R. & Dhawan, A. A flow cytometric method to assess nanoparticle uptake in bacteria. Cytometry A 79A, 707–712 (2011).
Dos Santos, T., Varela, J., Lynch, I., Salvati, A. & Dawson, K. A. quantitative assessment of the comparative nanoparticle-uptake efficiency of a range of cell lines. Small 7, 3341–3349 (2011).
Pratsinis, A., Hervella, P., Leroux, J. C., Pratsinis, S. E. & Sotiriou, G. A. Toxicity of silver nanoparticles in macrophages. Small 9, 2576–2584 (2013).
Wax, A. & Sokolov, K. Molecular imaging and darkfield microspectroscopy of live cells using gold plasmonic nanoparticles. Laser Photon. Rev. 3, 146–158 (2009).
Meyer, J. N. et al. Intracellular uptake and associated toxicity of silver nanoparticles in Caenorhabditis elegans. Aquat. Toxicol. 100, 140–150 (2010).
Fadeel, B. & Garcia-Bennett, A. E. Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv. Drug Del. Rev. 62, 362–374 (2010).
Salonen, J., Kaukonen, A. M., Hirvonen, J. & Lehto, V. P. Mesoporous silicon in drug delivery applications. J. Pharm. Sci. 97, 632–653 (2008).
Bimbo, L. M. et al. Drug permeation across intestinal epithelial cells using porous silicon nanoparticles. Biomaterials 32, 2625–2633 (2011).
Park, J.-H. et al. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nature Mater. 8, 331–336 (2009).
Wan, Y., Apostolou, S., Dronov, R., Kuss, B. & Voelcker, N. H. Cancer-targeting siRNA delivery from porous silicon nanoparticles. Nanomedicine 9, 2309–2321 (2014).
Kafshgari, M. H. et al. Oligonucleotide delivery by chitosan-functionalized porous silicon nanoparticles. Nano Res. 8, 2033–2046 (2015).
Cirtiu, C.-M., Fleury, N. & Chady, S. Assessing the fate of nanoparticles in biological fluids using SP-ICP-MS (2015); http://www.perkinelmer.com/CMSResources/Images/44-171044APP_012008_01-NexION-350S-Fate-of-NPs-in-Bio-Fluids.pdf
Peters, R. J. et al. Development and validation of single particle ICP-MS for sizing and quantitative determination of nano-silver in chicken meat. Anal. Bioanal. Chem. 406, 3875–3885 (2014). This paper is a good example of an integrated approach to the analysis of ENPs in complex matrices: from matrix-specific extraction methods to nano-specific single-particle analysis techniques.
Loeschner, K. et al. In-house validation of a method for determination of silver nanoparticles in chicken meat based on asymmetric flow field-flow fractionation and inductively coupled plasma mass spectrometric detection. Food Chem. 181, 78–84 (2015).
Grombe, R. et al. Production of reference materials for the detection and size determination of silica nanoparticles in tomato soup. Anal. Bioanal. Chem. 406, 3895–3907 (2014).
Tadjiki, S., Assemi, S., Deering, C., Veranth, J. & Miller, J. Detection, separation, and quantification of unlabeled silica nanoparticles in biological media using sedimentation field-flow fractionation. J. Nanopart. Res. 11, 981–988 (2009).
Miyashita, S.-i. et al. Time-resolved ICP-MS measurement: a new method for elemental and multiparametric analysis of single cells. Anal. Sci. 30, 219–224 (2014). This review elucidates technical principles and describes the challenges of TR ICP-MS applied to single-cell analysis, including the most recent developments of this technique.
De Jonge, M. D. et al. Quantitative 3D elemental microtomography of Cyclotella meneghiniana at 400-nm resolution. Proc. Natl Acad. Sci. USA 107, 15676–15680 (2010).
Ho, K.-S., Lui, K.-O., Lee, K.-H. & Chan, W.-T. Considerations of particle vaporization and analyte diffusion in single-particle inductively coupled plasma-mass spectrometry. Spectrochim. Acta Part B 89, 30–39 (2013).
Pace, H. E. et al. Determining transport efficiency for the purpose of counting and sizing nanoparticles via single particle inductively coupled plasma mass spectrometry. Anal. Chem. 83, 9361–9369 (2011).
Pace, H. E. et al. Single particle inductively coupled plasma-mass spectrometry: a performance evaluation and method comparison in the determination of nanoparticle size. Environ. Sci. Technol. 46, 12272–12280 (2012).
Reed, R. B., Higgins, C. P., Westerhoff, P., Tadjiki, S. & Ranville, J. F. Overcoming challenges in analysis of polydisperse metal-containing nanoparticles by single particle inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 27, 1093–1100 (2012).
Laborda, F., Jiménez-Lamana, J., Bolea, E. & Castillo, J. R. Selective identification, characterization and determination of dissolved silver(I) and silver nanoparticles based on single particle detection by inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 26, 1362–1371 (2011).
Laborda, F., Jiménez-Lamana, J., Bolea, E. & Castillo, J. R. Critical considerations for the determination of nanoparticle number concentrations, size and number size distributions by single particle ICP-MS. J. Anal. At. Spectrom. 28, 1220–1232 (2013).
Olesik, J. W. & Gray, P. J. Considerations for measurement of individual nanoparticles or microparticles by ICP-MS: determination of the number of particles and the analyte mass in each particle. J. Anal. At. Spectrom. 27, 1143–1155 (2012).
Mitrano, D. M. et al. Silver nanoparticle characterization using single particle ICP-MS (SP-ICP-MS) and asymmetrical flow field flow fractionation ICP-MS (AF4-ICP-MS). J. Anal. At. Spectrom. 27, 1131–1142 (2012).
Mitrano, D. M. et al. Detecting nanoparticulate silver using single-particle inductively coupled plasma–mass spectrometry. Environ. Toxicol. Chem. 31, 115–121 (2012). This article provides detailed insights into fundamental principles of single-particle ICP-MS, together with the example of the detection of silver nanoparticles in wastewater samples.
Mitrano, D. et al. Tracking dissolution of silver nanoparticles at environmentally relevant concentrations in laboratory, natural, and processed waters using single particle ICP-MS (spICP-MS). Env. Sci. Nano 1, 248–259 (2014).
Gray, E. P. et al. Extraction and analysis of silver and gold nanoparticles from biological tissues using single particle inductively coupled plasma mass spectrometry. Environ. Sci. Technol. 47, 14315–14323 (2013).
Hadioui, M., Leclerc, S. & Wilkinson, K. J. Multimethod quantification of Ag+ release from nanosilver. Talanta 105, 15–19 (2013).
Tuoriniemi, J., Cornelis, G. & Hassellöv, M. Size discrimination and detection capabilities of single-particle icpms for environmental analysis of silver nanoparticles. Anal. Chem. 84, 3965–3972 (2012).
Liu, J., Murphy, K. E., MacCuspie, R. I. & Winchester, M. R. Capabilities of single particle inductively coupled plasma mass spectrometry for the size measurement of nanoparticles: a case study on gold nanoparticles. Anal. Chem. 86, 3405–3414 (2014).
Telgmann, L., Metcalfe, C. & Hintelmann, H. Rapid size characterization of silver nanoparticles by single particle ICP-MS and isotope dilution. J. Anal. At. Spectrom. 29, 1265–1272 (2014).
Bi, X. et al. Quantitative resolution of nanoparticle sizes using single particle inductively coupled plasma mass spectrometry with the K-means clustering algorithm. J. Anal. At. Spectrom. 29, 1630–1639 (2014).
Striegel, A. M. & Brewer, A. K. Hydrodynamic chromatography. Annu. Rev. Anal. Chem. 5, 15–34 (2012).
Striegel, A. M. in Liquid Chromatography (eds Fanali, S. et al.) 193–223 (Elsevier, 2013).
Liu, F.-K. & Wei, G.-T. Effect of mobile-phase additives on separation of gold nanoparticles by size-exclusion chromatography. Chromatographia 59, 115–111 (2004).
Degueldre, C., Favarger, P. Y. & Wold, S. Gold colloid analysis by inductively coupled plasma–mass spectrometry in a single particle mode. Anal. Chim. Acta 555, 263–268 (2006).
Acknowledgements
The authors thank the South Australian Premier's Research and Industry Fund for a grant under the Collaboration Pathway program. We thank M. Cicera for preparing the figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Malysheva, A., Lombi, E. & Voelcker, N. Bridging the divide between human and environmental nanotoxicology. Nature Nanotech 10, 835–844 (2015). https://doi.org/10.1038/nnano.2015.224
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2015.224
This article is cited by
-
Considerations for designing preclinical cancer immune nanomedicine studies
Nature Nanotechnology (2021)
-
Engineered nanomaterial and their interactions with plant–soil system: a developmental journey and opposing facts
Nanotechnology for Environmental Engineering (2021)
-
A One Health approach to managing the applications and implications of nanotechnologies in agriculture
Nature Nanotechnology (2019)
-
Trade-off in membrane distillation with monolithic omniphobic membranes
Nature Communications (2019)
-
Multi-hierarchical profiling the structure-activity relationships of engineered nanomaterials at nano-bio interfaces
Nature Communications (2018)