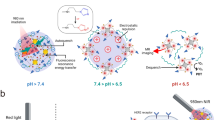
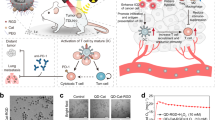
Abstract
The combination of light and photosensitizers for phototherapeutic interventions, such as photodynamic therapy, has transformed medicine and biology. However, the shallow penetration of light into tissues and the reliance on tissue oxygenation to generate cytotoxic radicals have limited the method to superficial or endoscope-accessible lesions. Here we report a way to overcome these limitations by using Cerenkov radiation from radionuclides to activate an oxygen-independent nanophotosensitizer, titanium dioxide (TiO2). We show that the administration of transferrin-coated TiO2 nanoparticles and clinically used radionuclides in mice and colocalization in tumours results in either complete tumour remission or an increase in their median survival. Histological analysis of tumour sections showed the selective destruction of cancerous cells and high numbers of tumour-infiltrating lymphocytes, which suggests that both free radicals and the activation of the immune system mediated the destruction. Our results offer a way to harness low-radiance-sensitive nanophotosensitizers to achieve depth-independent Cerenkov-radiation-mediated therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ethirajan, M., Chen, Y., Joshi, P. & Pandey, R. K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 40, 340–362 (2011).
Spring, B. Q. et al. Selective treatment and monitoring of disseminated cancer micrometastases in vivo using dual-function, activatable immunoconjugates. Proc. Natl Acad. Sci. USA 111, E993–E492 (2014).
Jelley, J. V. Cerenkov radiation and its applications. Br. J. Appl. Phys. 6, 227–232 (1955).
Robertson, R. et al. Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Phys. Med. Biol. 54, N355 (2009).
Cherry, S. & Dahlbom, M. in PET (ed. Phelps, M.) 1–117 (Springer, 2006).
Liu, H. et al. Molecular optical imaging with radioactive probes. PLoS ONE 5, e9470 (2010).
Spinelli, A. E. et al. Multispectral Cerenkov luminescence tomography for small animal optical imaging. Opt. Express 19, 12605–12618 (2011).
Kotagiri, N., Niedzwiedzki, D. M., Ohara, K. & Achilefu, S. Activatable probes based on distance-dependent luminescence associated with Cerenkov radiation. Angew. Chem. Int. Ed. 52, 7756–7760 (2013).
Sun, X. et al. Self-illuminating 64Cu-doped CdSe/ZnS nanocrystals for in vivo tumor imaging. J. Am. Chem. Soc. 136, 1706–1709 (2014).
Axelsson, J., Davis, S. C., Gladstone, D. J. & Pogue, B. W. Cerenkov emission induced by external beam radiation stimulates molecular fluorescence. Med. Phys. 38, 4127–4132 (2011).
Spinelli, A. E. et al. First human Cerenkography. J. Biomed. Opt. 18, 20502 (2013).
Thorek, D. L., Ogirala, A., Beattie, B. J. & Grimm, J. Quantitative imaging of disease signatures through radioactive decay signal conversion. Nature Med. 19, 1345–1350 (2013).
Linsebigler, A., Lu, G. & Yates, J. Photocatalysis on TiOn surfaces: principles, mechanisms, and selected results. Chem. Rev. 95, 735–758 (1995).
Schwarz, P. F. et al. A new method to determine the generation of hydroxyl radicals in illuminated TiO2 suspensions. J. Phys. Chem. B 101, 7127–7134 (1997).
Boehm, H. P. Acidic and basic properties of hydroxylated metal oxide surfaces. Discuss. Faraday Soc. 52, 264–275 (1971).
Mitchell, G. S., Gill, R. K., Boucher, D. L., Li, C. & Cherry, S. R. In vivo Cerenkov luminescence imaging: a new tool for molecular imaging. Philos. Trans. A 369, 4605–4619 (2011).
Huang, W., Lei, M., Huang, H., Chen, J. & Chen, H. Effect of polyethylene glycol on hydrophilic TiO2 films: porosity-driven superhydrophilicity. Surf. Coat. Technol. 204, 3954–3961 (2010).
Gatter, K. C., Brown, G., Trowbridge, I. S., Woolston, R. E. & Mason, D. Y. Transferrin receptors in human tissues: their distribution and possible clinical relevance. J. Clin. Pathol. 36, 539–545 (1983).
Ji, Z. et al. Dispersion and stability optimization of TiO2 nanoparticles in cell culture media. Environ. Sci. Technol. 44, 7309–7314 (2010).
Bowman, D. C. The amazingly versatile titanocene derivatives. J. Chem. Educ. 83, 735 (2006).
Clearfield, A., Warner, D. K., Saldarriaga-Molina, C. H., Ropal, R. & Bernal, I. Structural studies of (π-C5H5)2MX2 complexes and their derivatives. The structure of bis(π-cyclopentadienyl)titanium dichloride. Can. J. Chem. 53, 1622–1629 (1975).
Lümmen, G., Sperling, H., Luboldt, H., Otto, T. & Rübben, H. Phase II trial of titanocene dichloride in advanced renal-cell carcinoma. Cancer Chemother. Pharmacol. 42, 415–417 (1998).
Kröger, N., Kleeberg, U. R., Mross, K., Edler, L. & Hossfeld, D. K. Phase II clinical trial of titanocene dichloride in patients with metastatic breast cancer. Oncol. Res. Treat. 23, 60–62 (2000).
Davidenko, N., Garcia, O. & Sastre, R. The efficiency of titanocene as photoinitiator in the polymerization of dental formulations. J. Biomater. Sci. Polym. Ed. 14, 733–746 (2003).
Tehfe, M-A., Lalevée, J., Morlet-Savary, F., Graff, B. & Fouassier, J-P. On the use of bis(cyclopentadienyl)titanium(IV) dichloride in visible-light-induced ring-opening photopolymerization. Macromolecules 45, 356–361 (2011).
Brindley, P. B., Davies, A. G. & Hawari, J. A. A. An ESR study of the photolysis of dicyclopentadienyltitanium dichloride. J. Organomet. Chem. 250, 247–256 (1983).
Qian, Z. M., Li, H., Sun, H. & Ho, K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol. Rev. 54, 561–587 (2002).
Tijana, R., Nada, M. D., Adam, E. & Elena, R. in Handbook of Nanophysics 1–28 (CRC Press, 2010).
Paunesku, T. et al. Biology of TiO2-oligonucleotide nanocomposites. Nature Mater. 2, 343–346 (2003).
Zhao, J. et al. Titanium dioxide (TiO2) nanoparticles induce JB6 cell apoptosis through activation of the caspase-8/Bid and mitochondrial pathways. J. Toxicol. Environ. Health A 72, 1141–1149 (2009).
O'Connor, K. et al. Novel titanocene anti-cancer drugs and their effect on apoptosis and the apoptotic pathway in prostate cancer cells. Apoptosis 11, 1205–1214 (2006).
Kelloff, G. J. et al. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin. Cancer Res. 11, 2785–2808 (2005).
Heyne, B., Maurel, V. & Scaiano, J. C. Mechanism of action of sensors for reactive oxygen species based on fluorescein–phenol coupling: the case of 2-[6-(4′-hydroxy)phenoxy-3H-xanthen-3-on-9-yl]benzoic acid. Org. Biomol. Chem. 4, 802–807 (2006).
Rozhkova, E. A. et al. A high-performance nanobio photocatalyst for targeted brain cancer therapy. Nano Lett. 9, 3337–3342 (2009).
Misra, R. M., Bajaj, M. S. & Kale, V. P. Vasculogenic mimicry of HT1080 tumour cells in vivo: critical role of HIF-1α-neuropilin-1 axis. PLoS ONE 7, 21 (2012).
Dautry-Varsat, A., Ciechanover, A. & Lodish, H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc. Natl Acad. Sci. USA 80, 2258–2262 (1983).
Acknowledgements
Funding for this project was, in part, by grants from the US National Institutes of Health (NCI R01 CA171651 and P50 CA094056, NIBIB R01 EB008111 and R01 EB021048, and SIG S10 RR031625) and the National Science Foundation (CCF 0963742). We thank T. Voller and P. Eisenbeis for generously providing 64Cu, V. Sharma for technetium-99 m, W. Beatty and S. Greco for analysing histology sections, P. Gibbons, H. Wynder and J. Elsner for assistance with TEM imaging, and staff of the Siteman Cancer Center Small Animal microPET Facility for imaging with FDG-PET.
Author information
Authors and Affiliations
Contributions
N.K. and S.A. conceived and designed the experiments, analysed the data and wrote manuscript. N.K. performed most of the experiments. W.J.A. assisted in the animal study design and analysis and contributed to editing the final manuscript. G.P.S. assisted in cell studies, animal handling, histological section preparation and editing the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 542 kb)
Rights and permissions
About this article
Cite this article
Kotagiri, N., Sudlow, G., Akers, W. et al. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nature Nanotech 10, 370–379 (2015). https://doi.org/10.1038/nnano.2015.17
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2015.17
This article is cited by
-
New opportunities and old challenges in the clinical translation of nanotheranostics
Nature Reviews Materials (2023)
-
Antibacterial effect and photothermal sterilization of low dose two-dimensional vanadium carbide
Applied Physics A (2023)
-
Structure and Photosensitaizer Ability of Polymethine Dyes in Photodynamic Therapy: A Review
Theoretical and Experimental Chemistry (2023)
-
Chlorin e6-loaded goat milk-derived extracellular vesicles for Cerenkov luminescence-induced photodynamic therapy
European Journal of Nuclear Medicine and Molecular Imaging (2023)
-
Multimodal radiolabeled gold nanoparticle molecular probes: synthesis, imaging, and applications
Journal of Radioanalytical and Nuclear Chemistry (2023)