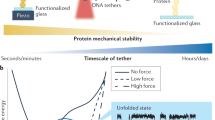
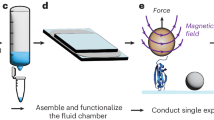
Abstract
Mechanical interactions are fundamental to biology. Mechanical forces of chemical origin determine motility and adhesion on the cellular scale, and govern transport and affinity on the molecular scale. Biological sensing in the mechanical domain provides unique opportunities to measure forces, displacements and mass changes from cellular and subcellular processes. Nanomechanical systems are particularly well matched in size with molecular interactions, and provide a basis for biological probes with single-molecule sensitivity. Here we review micro- and nanoscale biosensors, with a particular focus on fast mechanical biosensing in fluid by mass- and force-based methods, and the challenges presented by non-specific interactions. We explain the general issues that will be critical to the success of any type of next-generation mechanical biosensor, such as the need to improve intrinsic device performance, fabrication reproducibility and system integration. We also discuss the need for a greater understanding of analyte–sensor interactions on the nanoscale and of stochastic processes in the sensing environment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Braun, T. et al. Quantitative time-resolved measurement of membrane protein-ligand interactions using microcantilever array sensors. Nature Nanotech. 4, 179–185 (2009).
Phelan, M. L. & Nock, S. Generation of bioreagents for protein chips. Proteomics 3, 2123–2134 (2003).
von Muhlen, M. G., Brault, N. D., Knudsen, S. M., Jiang, S. & Manalis, S. R. Label-free biomarker sensing in undiluted serum with suspended microchannel resonators. Anal. Chem. 82, 1905–1910 (2010). This work is notable for the high sensitivity (300 pM) and fast response time (∼1 min).
Backmann, N. et al. A label-free immunosensor array using single-chain antibody fragments. Proc. Natl Acad. Sci. USA 102, 14587–14952 (2005). The authors use a microcantilever with static-mode deflection to achieve a sensitivity of ≈ 1 nM, and include a detailed discussion of device functionalization.
Waggoner, P. S., Varshney, M. & Craighead, H. G. Detection of prostate specific antigen with nanomechanical resonators. Lab Chip 9, 3095–3099 (2009). The authors report femtomolar detection from serum through fluid phase capture and detection in vacuo.
Ibach, H. The role of surface stress in reconstruction, epitaxial growth and stabilization of mesoscopic structures. Surf. Sci. 29, 193–263 (1997).
Wu, G. et al. Bioassay of prostrate-specific antigen (PSA) using microcantilevers. Nature Biotechnol. 19, 856–860 (2001).
Fritz, J. et al. Translating biomolecular recognition into nanomechanics. Science 288, 316–318 (2000).
Mertens, J. et al. Label-free detection of DNA hybridization based on hydration-induced tension in nucleic acid films. Nature Nanotech. 3, 302–307 (2008).
Zhang, J. et al. Rapid and label-free nanomechanical detection of biomarker transcripts in human RNA. Nature Nanotech. 1, 214–220 (2006).
Ndieyira, J. W. et al. Nanomechanical detection of antibiotic-mucopeptide binding in a model for superbug drug resistance. Nature Nanotech. 3, 691–696 (2008).
Braun, T. et al. Conformational change of bacteriorhodopsin quantitatively monitored by microcantilever sensors. Biophys. J. 90, 2970–2977 (2006).
Shu, W. et al. DNA molecular motor driven micromechanical cantilever arrays. J. Am. Chem. Soc. 127, 17054–17060 (2005).
Wee, K. W. et al. Novel electrical detection of label-free disease marker proteins using peizoresistive self-sensing micro-cantilevers. Biosens. Bioelectron. 20, 1932–1938 (2005).
Rasmussen, P. A., Thaysen, J., Hansen, O., Eriksen, S. C. & Boisen, A., Optimised cantilever biosensor with piezoresistive read-out. Ultramicroscopy 97, 371–376 (2003).
Stoney, G. G. The tension of metallic films deposited by electrolysis. Proc. R. Soc. Lond. A 82, 172–175 (1909).
Sader, J. E. Surface stress induced deflections of cantilever plates with applications to the atomic force microscopy: Rectangular plates. J. Appl. Phys. 89, 2911–2921 (2001).
Gfeller, K. Y., Nugaeva, N. & Hegner, M. Micromechanical oscillators as rapid biosensor for the detection of active growth of Escherichia coli. Biosens. Bioelectron. 21, 528–533 (2005).
Gfeller, K. Y., Nugaeva, N. & Hegner, M. Rapid biosensor for detection of antibiotic-selective growth of Escherichia coli. Appl. Environ. Microbiol. 71, 2626–2631 (2005). The growth and detection of E. coli was performed using dynamic mode microcantilevers: the sensitivity was ∼100 cells and the detection times were less than one hour.
Yang, Y. T., Callegari, C., Feng, X. L., Ekinci, K. L. & Roukes, M. L. Zeptogram-scale nanomechanical mass sensing. Nano Lett. 6, 583–586 (2006).
Jensen, K., Kim, K. & Zettl, A. An atomic-resolution nanomechanical mass sensor. Nature Nanotech. 3, 533–537 (2008).
Gupta, A., Akin, D. & Bashir, R. Single virus particle mass detection using microresonators with nanoscale thickness. Appl. Phys. Lett. 84, 1976–1978 (2004).
Ilic, B., Yang, Y. & Craighead, H. G. Virus detection using nanoelectromechanical devices. Appl. Phys. Lett. 85, 2604–2606 (2004).
Park, K. et al. Measurement of adherent cell mass and growth. Proc. Natl Acad. Sci. USA 107, 20691–20696 (2010).
Park, K. et al. Concentration, growth and mass measurements of mammalian cells on silicon cantilevers. Lab Chip 8, 1034–1041 (2008).
Sader, J. E. Frequency response of cantilever beams immersed in viscous fluids with applications to the atomic force microscope. J. Appl. Phys. 84, 64–76 (1998).
Van Eysden, C. A. & Sader, J. E. Frequency response of cantilever beams immersed in viscous fluids with applications to the atomic force microscope: Arbitrary mode order. J. Appl. Phys. 101, 044908 (2007). This paper includes a detailed theoretical analysis of the viscous damping of fluid-immersed cantilevers.
Braun, T. et al. Micromechanical mass sensors for biomolecular detection in a physiological environment. Phys. Rev. E 72, 031907 (2005). The authors use dynamic mode mass detection with microcantilevers to achieve a resolution of 7 ng.
Ghatkesar, M. K. et al. Higher modes of vibration increase mass sensitivity in nanomechanical microcantilevers. Nanotechnology 18, 445502 (2007).
Hwang, K. S. et al. In-situ quantitative analysis of a prostate-specific antigen (PSA) using a nanomechanical PZT cantilever. Lab Chip 4, 547–552 (2004).
Lee, J. H., Kim, T. S. & Yoon, K. H. Effect of mass and stress on resonant frequency shift of functionalized Pb(Zr0.52Ti0.48)O3 thin film microcantilever for the detection of C-reactive protein. Appl. Phys. Lett. 84, 3187–3189 (2004).
McFarland, A. W., Poggi, M. A., Doyle, M. J., Bottomley, L. A. & Colton, J. S. Influence of surface stress on the resonance behavior of microcantilevers. Appl. Phys. Lett. 87, 053505 (2005).
Lu, P., Lee, H. P., Lu, C. & O'Shea, S. J. Surface stress effects on the resonance properties of cantilever sensors. Phys. Rev. B 72, 085405 (2005).
Lachut, M. J. & Sader, J. E. Effect of surface stress on the stiffness of cantilever plates. Phys. Rev. Lett. 99, 206102 (2007).
Tamayo, J., Ramos, D., Mertens, J. & Calleja, M. Effect of the adsorbate stiffness on the resonance response of microcantilever sensors. Appl. Phys. Lett. 89, 224104 (2006).
Gupta, A. K. et al. Anomalous resonance in a nanomechanical biosensor. Proc. Natl Acad. Sci. USA 103, 13362–13367 (2006).
Burg, T. P. et al. Weighing of biomolecules, single cells and single nanoparticles in fluid. Nature 446, 1066–1069 (2007).
Barton, R. A. et al. Fabrication of a nanomechanical mass sensor containing a nanofluidic channel. Nano Lett. 10, 2058–2063 (2010).
Zuniga, C., Rinaldi, M. & Piazza, G. High frequency piezoelectric resonant nanochannels for bio-sensing applications in liquid environment. IEEE Sensors 2010 52–55 (November 2010).
Burg, T. P., Sader, J. E. & Manalis, S. R. Nonmonotonic energy dissipation in microfluidic resonators. Phys. Rev. Lett. 102, 228103 (2009).
Sader, J. E., Burg, T. P. & Manalis, S. R. Energy dissipation in microfluidic beam resonators. J. Fluid. Mech. 650, 215–250 (2010).
Bryan, A. K., Goranov, A., Amon, A. & Manalis, S. R. Measurement of mass, density, and volume during the cell cycle of yeast. Proc. Natl Acad. Sci. USA 107, 999–1004 (2010).
Godin, M. et al. Using buoyant mass to measure the growth of single cells. Nature Methods 7, 387–390 (2010).
Knudsen, S. M., von Muhlen, M. G., Schauer, D. B. & Manalis, S. R. Determination of bacterial antibiotic resistance based on osmotic shock response. Anal. Chem. 81, 7087–7090 (2009).
Arlett, J. L. & Roukes, M. L. Ultimate sensitivity limits for mass sensing with hollow micro/nanochannel resonators. J. Appl. Phys. 108, 084701 (2010).
Kim, N., Kim, D-K. & Cho, Y-J. Development of indirect-competitive quartz crystal microbalance immunosensor for C-reactive protein. Sensor. Actuat. B-Chem. 143, 444–448 (2009).
Kurosawa, S. et al. Evaluation of a high-affinity QCM immunosensor using antibody fragmentation and 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer. Biosens. Bioelectron. 20, 1134–1139 (2004).
Weizmann, Y., Patolsky, F. & Willner, I. Amplified detection of DNA and analysis of single-base mismatches by the catalyzed deposition of gold on Au-nanoparticles. Analyst 126, 1502–1504 (2001).
Armani, A. M., Kulkarni, R. P., Fraser, S. E. Flagan, R. C. & Vahala, K. J. Label-free, single-molecule detection with optical microcavities. Science 314, 783–787 (2007).
Arnold, S., Shopova, S. I. & Holler, S. Whispering gallery mode bio-sensor for label-free detection of single molecules: thermo-optic vs. reactive mechanism. Opt. Express 18, 281–287 (2010).
Washburn, A. L., Luchansky, M. S., Bowman, A. L. & Bailey, R. C. Quantitative, label-free detection of five protein biomarkers using muliplexed arrays of silicon photonic microring resonators. Anal. Chem. 82, 69–72 (2010). The authors report quantitative, parallel detection from five protein mixtures.
Luchansky, M. S. & Bailey, R. C. Silicon photonic microring resonators for quantitative cytokine detection and T-cell secretion anslysis. Anal. Chem. 82, 1975–1981 (2010).
Stern, E. et al. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature 445, 519–522 (2007).
Zheng, G., Gao, X. P. A. & Lieber, C. M. Frequency domain detection of biomolecules using silicon nanowire biosensors. Nano Lett. 10, 3179–3183 (2010).
Bunimovich, Y. L. et al. Quantitative real-time measurement of DNA hybridization with alkylated nonoxidized silicon nanowires in electrolyte solution. J. Am. Chem. Soc. 128, 16323–16331 (2006).
Gao, X. P. A., Zheng, G. & Lieber, C. M. Subthreshold regime has the optimal sensitivity for nanowire FET biosensors. Nano Lett. 10, 547–552 (2010).
Squires, T. M., Messinger, R. J. & Manalis, S. R. Making it stick: Convection reaction and diffusion in surface-based biosensors. Nature. Biotechnol. 26, 417–426 (2008). This review article contains a detailed kinetics analysis that is relevant to all biosensors.
Rich, R. & Myszka, D. G. Advances in surface plasmon resonance biosensor analysis. Curr. Opin. Biotechnol. 11, 54–61 (2000).
Skivesen, N. et al. Photonic-crystal waveguide biosensor. Opt. Express 15, 3169–3176 (2007).
Yao, X. et al. Sub-attomole oligonucleotide and p53 cDNA determinations via a high-resolution surface plasmon resonance combined with oligonucleotide-capped gold nanoparticle signal amplification. Anal. Biochem. 354, 220–228 (2006).
Cesaro-Tadic, S. et al. High-sensitivity miniaturized immunoassays for tumor necrosis factor α using microfluidic systems. Lab Chip 4, 563–569 (2004).
Fan, R. et al. Integrated barcode chips for rapid, multiplexed analysis of proteins in microliter quantities of blood. Nature Biotechnol. 26, 1373–1378 (2008).
Diercks, A. H. et al. A microfluidic device for multiplexed protein detection in nano-liter volumes. Anal. Biochem. 386, 30–35 (2009).
Harris, R. & Lohr, K. N. Screening for Prostate Cancer: An Update of the Evidence for the U. S. Preventitive Services Task Force. Ann. Intern. Med. 137, 915–916 (2002). See also: http://en.wikipedia.org/wiki/Prostate_specific_antigen
Carrano, J. Chemical and Biological Sensor Standards Study (DARPA, 2005). Available at http://go.nature.com/JNBVGN
Steward, C. C. & Steward, S. J. in Cytometry 3rd edn, Vol. 63 (ed. Darzynkiewicz, Z.) 223 (2001).
Konopka, K. & Neilands, J. B. Effect of serum albumin on siderophore-mediated utilization of transferring iron. Biochem. 10, 2122–2127 (1984).
Nair, P. R. & Alam, M. A. Theory of “selectivity” of label-free nanobiosensors: A geometro-physical perspective. J. Appl. Phys. 107, 064701 (2010).
Fan, R. et al. Integrated barcode chips for rapid, multiplexed analysis of proteins in microliter quantities of blood. Nature Biotechnol. 26, 1373–1378 (2008).
Stern, E. et al. Label-free biomarker detection from whole blood. Nature Nanotech. 5, 138–142 (2010). A two-stage detection approach leads to significantly enhanced specificity, enabling subpicomolar detection in whole blood with nanoribbon sensors.
Hirrlinger, J., Moeller, H., Kirchoff, F. & Dringen, R. Expression of multidrug resistance proteins (Mrps) in astrocytes of the mouse brain: A single cell RT-PCR study. Neurochem. Res. 30, 1237–1244 (2005).
Goluch, E. D. et al. A biobarcode assay for on-chip attomolar-sensitivity protein detection. Lab Chip 6, 1293–1299 (2006).
Grell, M., Wajant, H., Zimmermann, G. & Scheurich, P. The type 1 receptor (CD120a) is the high-affinity receptor for solube tumor necrosis factor. Proc. Natl Acad. Sci. USA 95, 570–575 (1998).
Florin, E. L., Moy, V. T. &. Gaub, H. E. Intermolecular forces and energies between ligands and receptors. Science 264, 415–417 (1994).
Fritz, J., Katopodis, A. G., Kolbinger, F. & Anselmetti, D. Force-mediated kinetics of single P-selectin/ligand complexes observed by atomic force microscopy. Proc. Natl Acad. Sci. USA 95, 12283–12288 (1998).
Merkel, R., Nassoy, P., Leung, A., Ritchie, K. & Evans, E. Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature 397, 50–53 (1999).
Wiita, A. P. et al. Probing the chemistry of thioredoxin catalysis with force. Nature 450, 124–127 (2007).
Fernandez, J. M. & Hongbin, L. Force spectroscopy monitors the folding trajectory of a single protein. Science 303, 1674–1678 (2004).
Tan, J. L. et al. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc. Natl Acad. Sci. USA 100, 1484–1489 (2003).
Guck, J. et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys. J. 88, 3689–3698 (2005).
Suresh, S. Biomechanics and biophysics of cancer cells. Acta Mater. 55, 3989–4014 (2007).
Lee, W., Fon, W., Axelrod, B. W. & Roukes, M. L. High-sensitivity microfluidic calorimeters for biological and chemical applications. Proc. Natl Acad. Sci. USA 106, 15225–15230 (2009).
Cross, S. E., Jin, Y-S., Rao, J. & Gimzewski, J. K. Nanomechanical analysis of cells from cancer patients. Nature Nanotech. 2, 780–783 (2007).
Brujić, J., Hermans, R. I., Walther, K. A. & Fernandez, J. M. Single-molecule force spectroscopy reveals signatures of glassy dynamics in the energy landscape of ubiquitin. Nature Phys. 2, 282–286 (2006).
Evans, E., Leung, A., Heinrich, V. & Zhu, Ch. Mechanical switching and coupling between two dissociation pathways in a P-selectin adhesion bond. Proc. Natl Acad. Sci. USA 101, 11281–11286 (2004).
Robert, P., Benoliel, A-M., Pierres, A. & Bongrand, P. What is the biological relevance of the specific bond properties revealed by single-molecule studies? J. Mol. Recognit. 20, 432–447 (2007).
Zhou, J. et al. Unbinding of the streptavidin-biotin complex by atomic force microscopy: a hybrid simulation study. J. Chem. Phys. 125, 104905 (2006).
Evans, E. A. & Calderwood, D. A. Forces and bond dynamics in cell adhesion. Science 316, 1148–1153 (2007).
Liphardt, J., Onoa, B., Smith, S. B., Tinoco, I. Jr & Bustamante, C. Reversible unfolding of single RNA molecules by mechanical force. Science 292, 733–737 (2001).
Krieg, M. et al. Tensile forces govern germ-layer organization in zebrafish. Nature Cell Biol. 10, 429–436 (2008).
Fierro, F. A. et al. BCR/ABL expression of myeloid progenitors increases beta1-integrin mediated adhesion to stromal cells. J. Mol. Biol. 377, 1082–1093 (2008).
Wjcikiewicz, E. et al. LFA-1 binding destabilizes the JAM-A homophilic interaction during leukocyte transmigration. Biophys. J. 96, 285–293 (2009).
Friedrichs, J., Helenius, J. & Müller, D. J. Stimulated single-cell force spectroscopy to quantify cell adhesion receptor crosstalk. Proteomics 10, 1455–1462 (2010).
Fon, W. C., Schwab, K. C., Worlock, J. M. & Roukes, M. L. Nanoscale, phonon-coupled calorimetry with sub-attojoule/Kelvin resolution. Nano Lett. 5, 1968–1971 (2005).
Ekinci, K. L. Electromechanical transducers at the nanoscale: actuation and sensing of motion in nanoelectromechanical systems (NEMS). Small 1, 786–797 (2005).
Carr, D. W., Evoy, S., Sekaric, L., Craighead, H. G. & Parpia, J. M. Measurement of mechanical resonance and losses in nanometer scale silicon wires. Appl. Phys. Lett. 75, 920–922 (1999).
Kouh, T., Karabacak, D., Kim, D. H. & Ekinci, K. L. Diffraction effects in optical interferometric displacement detection in nanoelectromechanical systems. Appl. Phys. Lett. 86, 013106 (2005).
Keeler, B. E. N., Carr, D. W., Sullivan, J. P., Friedmann, T. A. & Wendt, J. R. Experimental demonstration of a laterally deformable optical nanoelectromechanical system grating transducer. Opt. Lett. 29, 1182–1184 (2004).
Li, M., Pernice, W. H. P. & Tang, H. X. Broadband all-photonic transduction of nanocantilevers. Nature Nanotech. 4, 377–382 (2009).
Li, M. et al. Harnessing optical forces in integrated photonic circuits. Nature 467, 480–484 (2008).
Truitt, P. A., Hertzberg, J. B., Huang, C. C., Ekinci, K. L. & Schwab, K. C. Efficient and sensitive capacitive readout of nanomechanical resonator arrays. Nano Lett. 7, 120–126 (2007).
Sampathkumar, A., Murray, T. W. & Ekinci, K. L. Photothermal operation of high frequency nanoelectromechanical systems. Appl. Phys. Lett. 88, 223104 (2006).
Verbridge, S. S., Bellan, L. M., Parpia, J. M. & Craighead, H. G. Optically driven resonance of nanoscale flexural oscillators in liquid. Nano Lett. 6, 2109–2114 (2006).
Bargatin, I., Kokinsky, I. & Roukes, M. L. Efficient electrothermal actuation of multiple modes of high-frequency nanoelectromechanical resonators. Appl. Phys. Lett. 90, 093116 (2007).
Seo, J. H. & Brand, O. High Q-factor in-plane-mode resonant microsensor platform for gaseous/liquid environment. J. Microelectromech. Syst. 17, 483–493 (2008).
Piazza, G., Stephanou, P. J. & Pisano, A. P. Piezoelectric aluminum nitride vibrating contour-mode MEMS resonators. J. Microelectromech. Syst. 15, 1406–1418 (2006).
Karabalin, R. B. et al. Piezoelectric nanoelectromechanical resonators based on aluminum nitride thin films. Appl. Phys. Lett. 95, 103111 (2009).
Bargatin, I., Myers, E. B., Arlett, J., Gudlewski, B. & Roukes, M. L. Sensitive detection of nanomechanical motion using piezoresistive signal downmixing. Appl. Phys. Lett. 86, 133109 (2005).
Harley, J. A. & Kenny, T. W. High-sensitivity piezoeresistive cantilevers under 1000Å thick. Appl. Phys. Lett. 75, 289–291 (1999).
Arlett, J. L., Maloney, J. R., Gudlewski, B., Muluneh, M. & Roukes, M. L. Self-sensing micro- and nanocantilevers with attonewton-scale force resolution. Nano Lett. 6, 1000–1006 (2006).
Harley, J. A. & Kenny, T. W. 1/f noise considerations for the design and process optimization of piezoresistive cantilevers. J. Microelectromech. Syst. 9, 226–235 (2000).
Li, M., Tang, H. X. & Roukes, M. L. Ultra-sensitive NEMS-based cantilevers for sensing, scanned probe and very high-frequency applications. Nature Nanotech. 2, 114–120 (2007).
Nguyen, C. T-C. & Howe, R. T. An integrated CMOS micromechanical resonator high-Q oscillator. IEEE J. Solid-St. Circ. 34, 440–455 (1999).
Despont, M., Drechsler, U., Yu, R., Pogge, H. B. & Vettiger, P. Wafer-scale microdevice transfer/interconnect: its application in an AFM-based data-storage system. J. Microelectromech. Syst. 13, 895–901 (2004).
Thorsen, T., Maerkl, S. J. & Quake, S. R. Microfluidic large-scale integration. Science 298, 580–584 (2002).
Zhong, J. F. et al. A microfluidic processor for gene expression profiling of single human embryonic stem cells. Lab Chip 8, 68–74 (2008).
Gómez-Sjöberg, R., Leyrat, A. A., Pirone, D. M., Chen, C. S. & Quake, S. R. Versatile, fully automated, microfluidic cell culture system. Anal. Chem. 79, 8557–8563 (2007).
Mehta, A. I. et al. Biomarker amplification by serum carrier protein binding. Dis. Markers 19, 1–10 (2003).
Granger, J., Siddiqui, J., Copeland, S. & Remick, D. Albumin depletion of human plasma also removes low abundance proteins including the cytokines. Proteomics 5, 4713–4718 (2005).
Köster, S. et al. Drop-based microfluidic devices for encapsulation of single cells. Lab Chip 8, 1110–1115 (2008).
Pradines-Figueres, A. & Raetz, C. R. H. Processing and secretion of tumor necrosis factor α in endotoxin-treated Mono Mac 6 cells are dependent on phorbol myristate acetate. J. Biol. Chem. 267, 23261–23268 (1992).
Huber, F. et al. Analyzing gene expression using combined nanomechanical cantilever sensors. J. Phys.: Conf. Ser. 61, 450–453 (2007).
McKendry, R. et al. Multiple label-free biodetection and quantitative DNA-binding assays on a nanomechanical cantilever array. Proc. Natl Acad. Sci. USA 99, 9783–9788 (2002).
Huber, F., Hegner, M., Gerber, C., Güntherodt, H-J. & Lang, H. P. Label free analysis of transcription factors using microcantilever arrays. Biosens. Bioelectron. 21, 1599–1605 (2006).
Bietsch, A., Hegner, M., Lang, H. P. & Gerber, C. Inkjet deposition of alkanethiolate monolayers and DNA Oligonucleotides on gold: evaluation of spot uniformity by wet etching. Langmuir 20, 5119–5122 (2004).
Zhang, H., Lee, K. B., Li, Z. & Mirkin, C. A. Biofunctionalized nanoarrays of inorganic structures prepared by dip-pen nanolithography. Nanotechnology 14, 1113–1117 (2003).
Harper, J. C., Polsky, R., Dirk, S. M., Wheeler, D. R. & Brozik, S. M., Electroaddressable selective functionalization of electrode arrays: Catalytic NADH detection using ardyl diazonium modified gold electrodes. Electroanalysis 19, 1268–1274 (2007).
Zheng, G., Patolsky, F., Cui, Y., Wang, W. U. & Lieber, C. M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nature Biotechnol. 23, 1294–1301 (2005).
Lipsitz, R. Diagnostics at home: Pregnancy tests. Sci. Amer. 283, 110–111 (November 2000).
Washburn, A. L., Gunn, L. C. & Bailey, R. C. Label-free quantitation of a cancer biomarker in complex media using silicon photonic microring resonators. Anal. Chem. 81, 9499–9506 (2009).
Viani, M. B. et al. Small cantilevers for force spectroscopy of single molecules. J. Appl. Phys. 86, 2258–2262 (1999).
Arlett, J. L. et al. BioNEMS: Nanomechanical systems for single-molecule biophysics. Lect. Notes Phys. 711, 241–270 (2007).
Acknowledgements
The authors thank the Defense Advanced Research Projects Agency (HR00110610043 and N66001-08-1-2043) and the Fondation pour la Recherche et l'Enseignement Superieur for support. M.L.R. acknowledges a Director's Pioneer Award from the National Institutes of Health (1DP1OD006924). We also thank P. Puget for many discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Arlett, J., Myers, E. & Roukes, M. Comparative advantages of mechanical biosensors. Nature Nanotech 6, 203–215 (2011). https://doi.org/10.1038/nnano.2011.44
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2011.44
This article is cited by
-
Ultrasensitive detection of vital biomolecules based on a multi-purpose BioMEMS for Point of care testing: digoxin measurement as a case study
Scientific Reports (2024)
-
Design and parametric study of a tapered polymer-based suspended microfluidic channel for enhanced detection of biofluids and bioparticles
Microsystem Technologies (2023)
-
A brief review on novel biomarkers identified and advanced biosensing technologies developed for rapid diagnosis of Japanese Encephalitis Virus
Proceedings of the Indian National Science Academy (2022)
-
Avoiding transduction-induced heating in suspended microchannel resonators using piezoelectricity
Microsystems & Nanoengineering (2021)
-
Optomechanical crystals for spatial sensing of submicron sized particles
Scientific Reports (2021)