Abstract
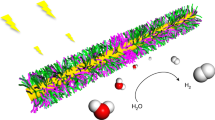
Over several billion years, cyanobacteria and plants have evolved highly organized photosynthetic systems to shuttle both electronic and chemical species for the efficient oxidation of water1. In a similar manner to reaction centres in natural photosystems, molecular2 and metal oxide3 catalysts have been used to photochemically oxidize water. However, the various approaches involving the molecular design of ligands4, surface modification5 and immobilization6,7 still have limitations in terms of catalytic efficiency and sustainability. Here, we demonstrate a biologically templated nanostructure for visible light-driven water oxidation that uses a genetically engineered M13 virus scaffold to mediate the co-assembly of zinc porphyrins (photosensitizer) and iridium oxide hydrosol clusters (catalyst). Porous polymer microgels are used as an immobilization matrix to improve the structural durability of the assembled nanostructures and to allow the materials to be recycled. Our results suggest that the biotemplated nanoscale assembly of functional components is a promising route to significantly improved photocatalytic water-splitting systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ferreira, K. N., Iverson, T. M., Maghlaoui, K., Barber, J. & Iwata, S. Architecture of the photosynthetic oxygen-evolving center. Science 303, 1831–1838 (2004).
Gersten, S. W., Samuels, G. J. & Meyer, T. J. Catalytic-oxidation of water by an oxo-bridged ruthenium dimer. J. Am. Chem. Soc. 104, 4029–4030 (1982).
Harriman, A., Pickering, I. J., Thomas, J. M. & Christensen, P. A. Metal-oxides as heterogeneous catalysts for oxygen evolution under photochemical conditions. J. Chem. Soc. Faraday Trans. I 84, 2795–2806 (1988).
McDaniel, N. D., Coughlin, F. J., Tinker, L. L. & Bernhard, S. Cyclometalated iridium(III) aquo complexes: efficient and tunable catalysts for the homogeneous oxidation of water. J. Am. Chem. Soc. 130, 210–217 (2008).
Hoertz, P. G., Kim, Y. I., Youngblood, W. J. & Mallouk, T. E. Bidentate dicarboxylate capping groups and photosensitizers control the size of IrO2 nanoparticle catalysts for water oxidation. J. Phys. Chem. B 111, 6845–6856 (2007).
Jiao, F. & Frei, H. Nanostructured cobalt oxide clusters in mesoporous silica as efficient oxygen-evolving catalysts. Angew. Chem. Int. Ed. 48, 1841–1844 (2009).
Hara, M., Lean, J. T. & Mallouk, T. E. Photocatalytic oxidation of water by silica-supported tris(4,4′-dialkyl-2,2′-bipyridyl)ruthenium polymeric sensitizers and colloidal iridium oxide. Chem. Mater. 13, 4668–4675 (2001).
Grätzel, M. Photoelectrochemical cells. Nature 414, 338–344 (2001).
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972).
Eisenberg, R. & Gray, H. B. Preface on making oxygen. Inorg. Chem. 47, 1697–1699 (2008).
Kanan, M. W. & Nocera, D. G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321, 1072–1075 (2008).
Turner, J. Oxygen catalysis: the other half of the equation. Nature Mater. 7, 770–771 (2008).
Meyer, T. J. Catalysis—the art of splitting water. Nature 451, 778–779 (2008).
Tagore, R., Crabtree, R. H. & Brudvig, G. W. Oxygen evolution catalysis by a dimanganese complex and its relation to photosynthetic water oxidation. Inorg. Chem. 47, 1815–1823 (2008).
Grätzel, M. Light-induced charge separation and water cleavage in microheterogeneous aqueous systems. Faraday Discuss. 70, 359–374 (1980).
Harriman, A., Nahor, G. S., Mosseri, S. & Neta, P. Iridium oxide hydrosols as catalysts for the decay of zinc porphyrin radical cations in water. J. Chem. Soc. Faraday Trans. I 84, 2821–2829 (1988).
Nahor, G. S., Mosseri, S., Neta, P. & Harriman, A. Polyelectrolyte-stabilized metal-oxide hydrosols as catalysts for the photooxidation of water by zinc porphyrins. J. Phys. Chem. 92, 4499–4504 (1988).
Gray, H. B. & Winkler, J. R. Long-range electron transfer. Proc. Natl Acad. Sci. USA 102, 3534–3539 (2005).
Nam, K. T. et al. Virus-enabled synthesis and assembly of nanowires for lithium ion battery electrodes. Science 312, 885–888 (2006).
Mao, C. B. et al. Virus-based toolkit for the directed synthesis of magnetic and semiconducting nanowires. Science 303, 213–217 (2004).
Lee, Y. J. et al. Fabricating genetically engineered high-power lithium-ion batteries using multiple virus genes. Science 324, 1051–1055 (2009).
Miller, R. A., Presley, A. D. & Francis, M. B. Self-assembling light-harvesting systems from synthetically modified tobacco mosaic virus coat proteins. J. Am. Chem. Soc. 129, 3104–3109 (2007).
Lee, S. K., Yun, D. S. & Belcher, A. M. Cobalt ion mediated self-assembly of genetically engineered bacteriophage for biomimetic Co–Pt hybrid material. Biomacromolecules 7, 14–17 (2006).
Glucksman, M. J., Bhattacharjee, S. & Makowski, L. Three-dimensional structure of a cloning vector. X-ray diffraction studies of filamentous bacteriophage M13 at 7 Å resolution. J. Mol. Biol. 226, 455–470 (1992).
Fudickar, W. et al. Fluorescence quenching and size selective heterodimerization of a porphyrin adsorbed to gold and embedded in rigid membrane gaps. J. Am. Chem. Soc. 121, 9539–9545 (1999).
Brixner, T. et al. Two-dimensional spectroscopy of electronic couplings in photosynthesis. Nature 434, 625–628 (2005).
Nam, Y. S. et al. Virus-templated assembly of porphyrins into light-harvesting nanoantennae. J. Am. Chem. Soc. 132, 1462–1463 (2010).
Morris, N. D., Suzuki, M. & Mallouk, T. E. Kinetics of electron transfer and oxygen evolution in the reaction of [Ru(bpy)3]3+ with colloidal iridium oxide. J. Phys. Chem. A 108, 9115–9119 (2004).
Kim, J. W., Utada, A. S., Fernandez-Nieves, A., Hu, Z. B. & Weitz, D. A. Fabrication of monodisperse gel shells and functional microgels in microfluidic devices. Angew. Chem. Int. Ed. 46, 1819–1822 (2007).
Soja, G. R. & Watson, D. F. TiO2-catalyzed photodegradation of porphyrins: mechanistic studies and application in monolayer photolithography. Langmuir 25, 5398–5403 (2009).
Acknowledgements
Y.S.N. would like to thank Y. Zhang for assistance with the scanning transmission electron microscopy, E.L. Shaw for help with the X-ray photoelectron spectroscopy, and K. Choi for experimental help and manuscript preparation. A.P.M thanks S. Cui for experimental help. This work was supported from Eni, S.p.A. (Italy) through the MIT Energy Initiative Program. We acknowledge the MIT Center for Materials Science and Engineering for use of microscopy and materials analysis facilities supported under grant no. DMR-9808941.
Author information
Authors and Affiliations
Contributions
Y.S.N. designed the study, prepared samples, collected data, performed oxygen evolution analyses, analysed data and wrote the manuscript. A.P.M. helped design experiments and analyse data, built the oxygen analysis system and edited the manuscript. D.L. fabricated the virus microgels, optimized the fabrication processes, and edited the manuscript. J.W.K. suggested, designed and fabricated the virus microgels. D.S.Y. carried out the biopanning experiment. H.P. performed oxygen evolution analyses, scopoletin assays and ICP-AES analyses. T.S.P. performed biological experiments and edited the manuscript. D.A.W. supervised the microgel research and edited the manuscript. A.M.B. designed the study, supervised the overall work and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1901 kb)
Rights and permissions
About this article
Cite this article
Nam, Y., Magyar, A., Lee, D. et al. Biologically templated photocatalytic nanostructures for sustained light-driven water oxidation. Nature Nanotech 5, 340–344 (2010). https://doi.org/10.1038/nnano.2010.57
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2010.57
This article is cited by
-
M13 Bacteriophage-Based Bio-nano Systems for Bioapplication
BioChip Journal (2022)
-
Advances in photo-enzymatic-coupling catalysis system
Systems Microbiology and Biomanufacturing (2021)
-
Virus lasers for biological detection
Nature Communications (2019)
-
Probing molecular mechanisms of M13 bacteriophage adhesion
Communications Chemistry (2019)
-
Comparing proteins and nucleic acids for next-generation biomolecular engineering
Nature Reviews Chemistry (2018)