Abstract
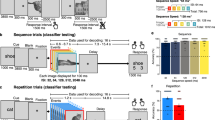
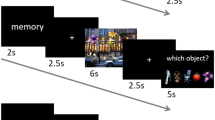
We conducted two event-related functional magnetic resonance imaging (fMRI) experiments to investigate the neural substrates of visual object recognition in humans. We used a repetition-priming method with visual stimuli recurring at unpredictable intervals, either with the same appearance or with changes in size, viewpoint or exemplar. Lateral occipital and posterior inferior temporal cortex showed lower activity for repetitions of both real and non-sense objects; fusiform and left inferior frontal regions showed decreases for repetitions of only real objects. Repetition of different exemplars with the same name affected only the left inferior frontal cortex. Crucially, priming-induced decreases in activity of the right fusiform cortex depended on whether the three-dimensional objects were repeated with the same viewpoint, regardless of whether retinal image size changed; left fusiform decreases were independent of both viewpoint and size. These data show that dissociable subsystems in ventral visual cortex maintain distinct view-dependent and view-invariant object representations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Biederman, I. Recognition-by-components: a theory of human image understanding. Psychol. Rev. 94, 115–147 (1987).
Biederman, I. & Gerhardstein, P.C. Recognizing depth-rotated objects: evidence and conditions for three-dimensional viewpoint invariance. J. Exp. Psychol. Hum. Percept. Perform. 19, 1162–1182 (1993).
Bulthoff, H.H., Edelman, S.Y. & Tarr, M.J. How are three-dimensional objects represented in the brain? Cereb. Cortex 5, 247–260 (1995).
Tarr, M.J. & Bulthoff, H.H. Image-based object recognition in man, monkey and machine. Cognition 67, 1–20 (1998).
Wallis, G. & Bulthoff, H. Learning to recognize objects. Trends Cogn. Sci. 3, 22–31 (1999).
Marr, D. Vision (W. H. Freeman, San Francisco, 1982).
Tarr, M.J., Williams, P., Hayward, W.G. & Gauthier, I. Three-dimensional object recognition is viewpoint dependent. Nat. Neurosci. 1, 275–277 (1998).
Ellis, R., Allport, D.A., Humphreys, G.W. & Collis, J. Varieties of object constancy. Q. J. Exp. Psychol. A 41, 775–796 (1989).
Biederman, I. & Bar, M. One-shot viewpoint invariance in matching novel objects. Vision Res. 39, 2885–2899 (1999).
Warrington, E.K. & Taylor, A.M. Two categorical stages of object recognition. Perception 7, 695–705 (1978).
Grüsser, O.J. & Landis, T. Visual agnosia and other disturbances of visual perception and cognition(ed. Cronly-Dillon, J. R.) (MacMillan, London, 1991).
Turnbull, O.H., Carey, D.P. & McCarthy, R.A. The neuropsychology of object constancy. J. Int. Neuropsychol. Soc. 3, 288–298 (1997).
Lueschow, A., Miller, E.K. & Desimone, R. Inferior temporal mechanisms for invariant object recognition. Cereb. Cortex 4, 523–531 (1994).
Booth, M.C. & Rolls, E.T. View-invariant representations of familiar objects by neurons in the inferior temporal visual cortex. Cereb. Cortex 8, 510–523 (1998).
Logothetis, N.K., Pauls, J., Bulthoff, H.H. & Poggio, T. View-dependent object recognition by monkeys. Curr. Biol. 4, 401–414 (1994).
Ashbridge, E., Perrett, D.I., Oram, M.W. & Jellema, T. Effect of image orientation and size on object recognition: responses of single units in the macaque monkey temporal cortex. Cognit. Neuropsychol. 17, 13–34 (2000).
Schacter, D.L. & Buckner, R.L. Priming and the brain. Neuron 20, 185–195 (1998).
Biederman, I. & Cooper, E.E. Evidence for complete translational and reflectional invariance in visual object priming. Perception 20, 585–593 (1991).
Furmanski, C.S. & Engel, S.A. Perceptual learning in object recognition: object specificity and size invariance. Vision Res. 40, 473–484 (2000).
Fiser, J. & Biederman, I. Invariance of long-term visual priming to scale, reflection, translation, and hemisphere. Vision Res. 41, 221–234 (2001).
Cave, C.B., Bost, P.R. & Cobb, R.E. Effects of color and pattern on implicit and explicit picture memory. J. Exp. Psychol. Learn. Mem. Cogn. 22, 639–653 (1996).
Desimone, R. Neural mechanisms for visual memory and their role in attention. Proc. Natl. Acad. Sci. USA 93, 13494–13499 (1996).
Blaxton, T.A. et al. Functional mapping of human memory using PET: comparisons of conceptual and perceptual tasks. Can. J. Exp. Psychol. 50, 42–56 (1996).
Buckner, R.L., Koutstaal, W., Schacter, D.L. & Rosen, B.R. Functional MRI evidence for a role of frontal and inferior temporal cortex in amodal components of priming. Brain 3, 620–640 (2000).
Henson, R., Shallice, T. & Dolan, R. Neuroimaging evidence for dissociable forms of repetition priming. Science 287, 1269–1272 (2000).
Buckner, R.L. et al. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron 20, 285–296 (1998).
James, T.W., Humphrey, G.K., Gati, J.S., Menon, R.S. & Goodale, M.A. Repetition priming and the time course of object recognition: an fMRI study. Neuroreport 10, 1019–1023 (1999).
Koutstaal, W. et al. Perceptual specificity in visual object priming: functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia 39, 184–199 (2001).
van Turennout, M., Ellmore, T. & Martin, A. Long-lasting cortical plasticity in the object naming system. Nat. Neurosci 3, 1329–1334 (2000).
Schacter, D.L. et al. Brain regions associated with retrieval of structurally coherent visual information. Nature 376, 587–590 (1995).
Kourtzi, Z. & Kanwisher, N. Cortical regions involved in perceiving object shape. J. Neurosci. 20, 3310–3318 (2000).
Kourtzi, Z. & Kanwisher, N. Representation of perceived object shape by the human lateral occipital complex. Science 293, 1506–1509 (2001).
Grill-Spector, K. et al. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron 24, 187–203 (1999).
Grill-Spector, K., Kourtzi, Z. & Kanwisher, N. The lateral occipital complex and its role in object recognition. Vision Res. 41, 1409–1422 (2001).
Bentin, S. & Moscovitch, M. The time course of repetition effects for words and unfamiliar faces. J. Exp. Psychol. Gen. 117, 148–60 (1988).
Nagy, M.E. & Rugg, M.D. Modulation of event-related potentials by word repetition: the effects of inter-item lag. Psychophysiology 26, 431–436 (1989).
Friston, K.J., Holmes, A.P., Price, C.J., Buchel, C. & Worsley, K.J. Multisubject fMRI studies and conjunction analyses. Neuroimage 10, 385–396 (1999).
Malach, R. et al. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc. Natl. Acad. Sci. USA 92, 8135–8139 (1995).
Wagner, A.D., Koutstaal, W., Maril, A., Schacter, D.L. & Buckner, R.L. Task-specific repetition priming in left inferior prefrontal cortex. Cereb. Cortex 10, 1176–1184 (2000).
Poggio, T. & Edelman, S. A network that learns to recognize three-dimensional objects.Nature 343, 263–266 (1990).
Grill-Spector, K., Kushnir, T., Edelman, S., Itzchak, Y. & Malach, R. Cue-invariant activation in object-related areas of the human occipital lobe. Neuron 21, 191–202 (1998).
Lerner, Y., Hendler, T., Ben-Bashat, D., Harel, M. & Malach, R. A hierarchical axis of object processing stages in the human visual cortex.Cereb. Cortex 11, 287–297 (2001).
Riesenhuber, M. & Poggio, T. Hierarchical models of object recognition in cortex. Nat Neurosci 2, 1019–1025 (1999).
Burgund, E.D. & Marsolek, C.J. Viewpoint-invariant and viewpoint-dependent object recognition in dissociable neural subsystems. Psychonom. Bull. Rev. 7, 480–489 (2000).
Tsunoda, K., Yamane, Y., Nishizaki, M. & Tanifuji, M. Complex objects are represented in macaque inferotemporal cortex by the combination of feature columns. Nat. Neurosci. 4, 832–838 (2001).
Farah, M.J. Visual Agnosia: Disorders of Object Recognition and What They Tell Us About Normal Vision (MIT Press, Cambridge, Massachusetts, 1990).
Wallis, G. & Rolls, E.T. Invariant face and object recognition in the visual system. Prog. Neurobiol. 51, 167–194 (1997).
Tanaka, K. Inferotemporal cortex and object vision. Annu. Rev. Neurosci. 19, 109–139 (1996).
Friston, K.J. et al. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 2, 189–210 (1995).
Worsley, K.J. et al. A unified statistical approach for determining significant signals in images of cerebral activation. Hum. Brain Mapp. 4, 58–73 (1996).
Acknowledgements
We thank R. Ellis, M. Tucker and M. Tarr for some of the stimuli, and the radiographers at the Functional Imaging Laboratory for technical assistance. This work was supported by Wellcome Programme grants to R.J.D. and J.D., a Wellcome Fellowship to R.N.H. and a Medical Research Council (UK) Co-operative Grant for 'Analysis of cognitive impairment and imaging of cognition' at University College London. J.D. holds a Royal Society–Wolfson Research Merit Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Vuilleumier, P., Henson, R., Driver, J. et al. Multiple levels of visual object constancy revealed by event-related fMRI of repetition priming. Nat Neurosci 5, 491–499 (2002). https://doi.org/10.1038/nn839
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn839
This article is cited by
-
Banknote authenticity is signalled by rapid neural responses
Scientific Reports (2022)
-
The Ties that Bind: Agnosia, Neglect and Selective Attention to Visual Scale
Current Neurology and Neuroscience Reports (2021)
-
Object responses are highly malleable, rather than invariant, with changes in object appearance
Scientific Reports (2020)
-
Endogenous activity modulates stimulus and circuit-specific neural tuning and predicts perceptual behavior
Nature Communications (2020)
-
David and Goliath—size does matter: size modulates feature–response binding of irrelevant features
Psychological Research (2020)