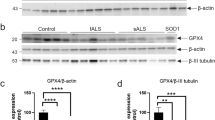
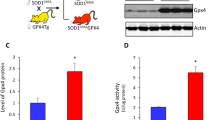
Abstract
Copper-mediated oxidative damage is proposed to play a critical role in the pathogenesis of Cu/Zn superoxide dismutase (SOD1)–linked familial amyotrophic lateral sclerosis (FALS). We tested this hypothesis by ablating the gene encoding the copper chaperone for SOD1 (CCS) in a series of FALS-linked SOD1 mutant mice. Metabolic 64Cu labeling in SOD1-mutant mice lacking the CCS showed that the incorporation of copper into mutant SOD1 was significantly diminished in the absence of CCS. Motor neurons in CCS−/− mice showed increased rate of death after facial nerve axotomy, a response documented for SOD1−/− mice. Thus, CCS is necessary for the efficient incorporation of copper into SOD1 in motor neurons. Although the absence of CCS led to a significant reduction in the amount of copper-loaded mutant SOD1, however, it did not modify the onset and progression of motor neuron disease in SOD1-mutant mice. Hence, CCS-dependent copper loading of mutant SOD1 plays no role in the pathogenesis of motor neuron disease in these mouse models.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rosen, D. R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993).
Deng, H. X. et al. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science 261, 1047–1051 (1993).
Wong, P. C. et al. An adverse property of a familial ALS–linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron 14, 1105–1116 (1995).
Gurney, M. E. et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994).
Ripps, M. E., Huntley, G. W., Hof, P. R., Morrison, J. H. & Gordon, J. W. Transgenic mice expressing an altered murine superoxide dismutase gene provide an animal model of amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. 92, 689–693 (1995).
Bruijn, L. I. et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18, 327–338 (1997).
Julien, J.-P. Amyotrophic lateral sclerosis. Unfolding the toxicity of the misfolded. Cell 104, 581–591 (2001).
Cleveland, D. W. & Rothstein, J. D. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat. Rev. Neurosci. 2, 806–819 (2001).
Estevez, A. G. et al. Induction of nitric oxide–dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science 286, 2498–2500 (1999).
Wiedau-Pazos, M. et al. Altered reactivity of superoxide dismutase in familial amyotrophic lateral sclerosis. Science 271, 515–518 (1996).
Beckman, J. S., Carson, M., Smith, C. D. & Koppenol, W. H. ALS, SOD and peroxynitrite. Nature 364, 584 (1993).
Rae, T. D., Schmidt, P. J., Pufahl, R. A., Culotta, V. C. & O'Halloran, T. V. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284, 805–808 (1999).
Kuo, Y. M., Zhou, B., Cosco, D. & Gitschier, J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc. Natl. Acad. Sci. USA 98, 6836–6841 (2001).
Lee, J., Prohaska, J. R. & Thiele, D. J. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc. Natl. Acad. Sci. USA 98, 6842–6847 (2001).
Culotta, V. C. et al. Intracellular pathways of copper trafficking in yeast and humans. Adv. Exp. Med. Biol. 448, 247–254 (1999).
Culotta, V. C. et al. The copper chaperone for superoxide dismutase. J. Biol. Chem. 272, 23469–23472 (1997).
Torres, A. S., Petri, V., Rae, T. D. & O'Halloran, T. V. Copper-stabilized heterodimer of the yCCS metallochaperone and its target superoxide dismutase. J. Biol. Chem. 276, 38410–38416 (2001).
Wong, P. C. et al. Copper chaperone for superoxide dismutase is essential to activate mammalian Cu/Zn superoxide dismutase. Proc. Natl. Acad. Sci. USA 97, 2886–2891 (2000).
Crapo, J., McCord, J. M. & Fridovich, I. Preparation and assay of superoxide dismutases. Methods Enzymol. 53, 382–393 (1978).
Reaume, A. G. et al. Motor neurons in Cu/Zn superoxide dismutase–deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genetics 13, 43–47 (1996).
Mouton, P. R. et al. Cognitive decline strongly correlates with cortical atrophy in Alzheimer's dementia. Neurobiol. Aging 19, 371–377 (1998).
Lyons, W. E. et al. Neuronal regeneration enhances the expression of the immunophilin FKBP-12. J. Neurosci. 15, 2985–2994 (1995).
Williamson, T. L. et al. Toxicity of ALS-linked SOD1 mutants. Science 288, 399 (2000).
Borchelt, D. R. et al. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc. Natl. Acad. Sci. USA 91, 8292–8296 (1994).
Corson, L. B, Strain, J. J., Culotta, V. C. & Cleveland, D. W. Chaperone-facilitated copper binding is a property common to several classes of familial amyotrophic lateral sclerosis–linked superoxide dismutase mutants. Proc. Natl. Acad. Sci. USA 95, 6361–6366 (1998).
Crow, J. P. et al. Superoxide dismutase catalyzes nitration of tyrosines by peroxynitrite in the rod and head domains of neurofilament-L. J. Neurochem. 69, 1945–1953 (1997).
Doroudchi, M. M., Minotti, S., Figlewicz, D. A. & Durham, H. D. Nitrotyrosination contributes minimally to toxicity of mutant SOD1 associated with ALS. Neuroreport 12, 1239–1243 (2001).
Facchinetti, F. et al. Lack of involvement of neuronal nitric oxide synthase in the pathogenesis of a transgenic mouse model of familial amyotrophic lateral sclerosis. Neuroscience 90, 1483–1492 (1999).
Yim, M. B. et al. A gain-of-function of an amyotrophic lateral sclerosis–associated Cu,Zn-superoxide dismutase mutant: an enhancement of free radical formation due to a decrease in Km for hydrogen peroxide. Proc. Natl. Acad. Sci. USA 93, 5709–5714 (1996).
Yim, H. S. et al. A familial amyotrophic lateral sclerosis–associated A4V Cu,Zn-superoxide dismutase mutant has a lower Km for hydrogen peroxide. Correlation between clinical severity and the Km value. J. Biol. Chem. 272, 8861–8863 (1997).
Singh, R. J. et al. Reexamination of the mechanism of hydroxyl radical adducts formed from the reaction between familial amyotrophic lateral sclerosis–associated Cu,Zn superoxide dismutase mutants and H2O2 . Proc. Natl. Acad. Sci. USA 95, 6675–6680 (1998).
Sankarapandi, S. & Zweier, J. L. Evidence against the generation of free hydroxyl radicals from the interaction of copper, zinc-superoxide dismutase and hydrogen peroxide. J. Biol. Chem. 274, 34576–34583 (1999).
Goto, J. J. et al. Loss of in vitro metal ion binding specificity in mutant copper–zinc superoxide dismutases associated with familial amyotrophic lateral sclerosis. J. Biol. Chem. 275, 1007–1014 (2000).
Liu, H. et al. Copper2+ binding to the surface residue cysteine 111 of His46Arg human copper–zinc superoxide dismutase, a familial amyotrophic lateral sclerosis mutant. Biochemistry 39, 8125–8132 (2000).
Martin, L. J. Neuronal death in amyotrophic lateral sclerosis is apoptosis: possible contribution of a programmed cell death mechanism. J. Neuropathol. Exp. Neurol. 58, 459–471 (1999).
Li, M. et al. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science 288, 335–339 (2000).
Pasinelli, P., Houseweart, M. K., Brown, R. H. Jr. & Cleveland, D. W. Caspase-1 and -3 are sequentially activated in motor neuron death in Cu,Zn superoxide dismutase–mediated familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 97, 13901–13906 (2000).
Bruijn, L. I. et al. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science 281, 1851–1854 (1998).
Johnston, J. A, Dalton, M. J., Gurney, M. E. & Kopito, R. R. Formation of high molecular weight complexes of mutant Cu,Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 97, 12571–12576 (2000).
Beaulieu, J. M., Jacomy, H. & Julien, J. P. Formation of intermediate filament protein aggregates with disparate effects in two transgenic mouse models lacking the neurofilament light subunit. J. Neurosci. 20, 5321–5328 (2000).
Sunderman, F. W. Jr. & Nomoto, S. Measurement of human serum ceruloplasmin by its p-phenylenediamine oxidase activity. Clin. Chem. 11, 903–910 (1970).
Prohaska, J. R & Bailey, W. R. Persistent regional changes in brain copper, cuproenzymes and catecholamines following perinatal copper deficiency in mice. J. Nutr. 123, 1226–1234 (1993).
Acknowledgements
We thank G. Cristostomo for support in histology, E. Corpus for mouse maintenance, L. Jensen for assistance in copper content determination, T. O'Halloran for apoSOD1 and D. Borchelt and V. Culotta for discussions. This work has been supported by grants from the National Institute of Health (P.C.W., D.L.P., D.W.C. and J.D.G.), Amyotrophic Lateral Sclerosis Association (P.C.W.) and The Spinal Cord Research Foundation (J.L.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Subramaniam, J., Lyons, W., Liu, J. et al. Mutant SOD1 causes motor neuron disease independent of copper chaperone–mediated copper loading. Nat Neurosci 5, 301–307 (2002). https://doi.org/10.1038/nn823
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn823
This article is cited by
-
UXT chaperone prevents proteotoxicity by acting as an autophagy adaptor for p62-dependent aggrephagy
Nature Communications (2021)
-
Exploring the Extended Biological Functions of the Human Copper Chaperone of Superoxide Dismutase 1
The Protein Journal (2019)
-
Redox metals homeostasis in multiple sclerosis and amyotrophic lateral sclerosis: a review
Cell Death & Disease (2018)
-
A faulty interaction between SOD1 and hCCS in neurodegenerative disease
Scientific Reports (2016)
-
A random set scoring model for prioritization of disease candidate genes using protein complexes and data-mining of GeneRIF, OMIM and PubMed records
BMC Bioinformatics (2014)