Abstract
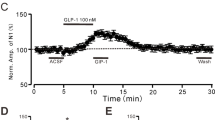
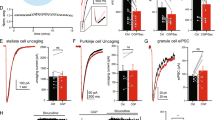
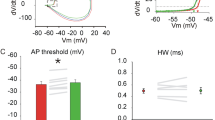
Metabotropic γ-aminobutyric acid type B (GABAB) and glutamate receptors (mGluRs) are postsynaptically co-expressed at cerebellar parallel fiber (PF)–Purkinje cell (PC) excitatory synapses, but their functional interactions are unclear. We found that mGluR1 agonist-induced currents and [Ca2+]i increases in PCs were enhanced following co-activation of GABAB receptors. A GABAB antagonist and a G-protein uncoupler suppressed these effects. Low-concentration baclofen, a GABAB agonist, augmented mGluR1-mediated excitatory synaptic current produced by stimulating PFs. These results indicate that postsynaptic GABAB receptors functionally interact with mGluR1 and enhance mGluR1-mediated excitatory transmission at PF–PC synapses. The interaction between the two types of metabotropic receptors provides a likely mechanism for regulating cerebellar synaptic plasticity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Bowery, N. G. GABAB receptor pharmacology. Annu. Rev. Pharmacol. Toxicol. 33, 109–147 (1993).
Barnard, E. A. et al. Subtypes of GABAA receptors: classification on the basis of subunit structure and receptor function. Pharmacol. Rev. 50, 291–313 (1998).
Kaupmann, K. et al. Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature 386, 239–246 (1997).
Jones, K. A. et al. GABAB receptors function as a heteromeric assembly of the subunits GABABR1 and GABABR2. Nature 396, 674–679 (1998).
Kaupmann, K. et al. GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature 396, 683–687 (1998).
Kuner, R. et al. Role of heteromer formation in GABAB receptor function. Science 283, 74–77 (1999).
Thompson, S. M., Capogna, M. & Scanziani, M. Presynaptic inhibition in the hippocampus. Trends Neurosci. 16, 222–227 (1993).
Wu, L. G. & Saggau, P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 20, 204–212 (1997).
Sodickson, D. L. & Bean, B. P. GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J. Neurosci. 16, 6374–6385 (1996).
Luscher, C., Jan, L. Y., Stoffel, M., Malenka, R. C. & Nicoll, R. A. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 19, 687–695 (1997).
Mintz, I. M. & Bean, B. P. GABAB receptor inhibition of P-type Ca2+ channels in central neurons. Neuron 10, 889–898 (1993).
Kerr, D. I. & Ong, J. GABAB receptors. Pharmacol. Ther. 67, 187–246 (1995).
Martinelli, G. P., Holstein, G. R., Pasik, P. & Cohen, B. Monoclonal antibodies for ultrastructural visualization of L-baclofen-sensitive GABAB receptor sites. Neuroscience 46, 23–33 (1992).
Turgeon, S. M. & Albin, R. L. Pharmacology, distribution, cellular localization, and development of GABAB binding in rodent cerebellum. Neuroscience 55, 311–323 (1993).
Fritschy, J. M. et al. GABAB-receptor splice variants GB1a and GB1b in rat brain: developmental regulation, cellular distribution and extrasynaptic localization. Eur. J. Neurosci. 11, 761–768 (1999).
Kawaguchi, S. & Hirano, T. Suppression of inhibitory synaptic potentiation by presynaptic activity through postsynaptic GABAB receptors in a Purkinje neuron. Neuron 27, 339–347 (2000).
Ito, M. Long-term depression. Annu. Rev. Neurosci. 12, 85–102 (1989).
Linden, D. J. & Connor, J. A. Long-term synaptic depression. Annu. Rev. Neurosci. 18, 319–357 (1995).
Conquet, F. et al. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature 372, 237–243 (1994).
Ichise, T. et al. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science 288, 1832–1835 (2000).
Nusser, Z., Mulvihill, E., Streit, P. & Somogyi, P. Subsynaptic segregation of metabotropic and ionotropic glutamate receptors as revealed by immunogold localization. Neuroscience 61, 421–427 (1994).
Luján, R., Roberts, J. D., Shigemoto, R., Ohishi, H. & Somogyi, P. Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1 alpha, mGluR2 and mGluR5, relative to neurotransmitter release sites. J. Chem. Neuroanat. 13, 219–241 (1997).
Mateos, J. M. et al. Immunolocalization of the mGluR1b splice variant of the metabotropic glutamate receptor 1 at parallel fiber–Purkinje cell synapses in the rat cerebellar cortex. J. Neurochem. 74, 1301–1309 (2000).
Staub, C., Vranesic, I. & Knöpfel, T. Responses to metabotropic glutamate receptor activation in cerebellar Purkinje cells: induction of an inward current. Eur. J. Neurosci. 4, 832–839 (1992).
Linden, D. J., Smeyne, M. & Connor, J. A. Trans-ACPD, a metabotropic receptor agonist, produces calcium mobilization and an inward current in cultured cerebellar Purkinje neurons. J. Neurophysiol. 71, 1992–1998 (1994).
Hirono, M., Konishi, S. & Yoshioka, T. Phospholipase C-independent group I metabotropic glutamate receptor-mediated inward current in mouse Purkinje cells. Biochem. Biophys. Res. Commun. 251, 753–758 (1998).
Goodman, R. R., Kuhar, M. J., Hester, L. & Snyder, S. H. Adenosine receptors: autoradiographic evidence for their location on axon terminals of excitatory neurons. Science 220, 967–969 (1983).
Jaarsma, D., Levey, A. I., Frostholm, A., Rotter, A. & Voogd, J. Light-microscopic distribution and parasagittal organisation of muscarinic receptors in rabbit cerebellar cortex. J. Chem. Neuroanat. 9, 241–259 (1995).
Miquel, M. C. et al. Postnatal development and localization of 5-HT1A receptor mRNA in rat forebrain and cerebellum. Brain Res. Dev. Brain Res. 80, 149–157 (1994).
Konishi, S. & Mitoma, H. in The Role of Adenosine in the Nervous System (ed. Okada, Y.) 89–95 (Elsevier, New York, 1997).
Mitoma, H. & Konishi, S. Monoaminergic long-term facilitation of GABA-mediated inhibitory transmission at cerebellar synapses. Neuroscience 88, 871–883 (1999).
Yamada, J., Saitow, F., Satake, S., Kiyohara, T. & Konishi, S. GABAB receptor-mediated presynaptic inhibition of glutamatergic and GABAergic transmission in the basolateral amygdala. Neuropharmacology 38, 1743–1753 (1999).
Takahashi, M., Kovalchuk, Y. & Attwell, D. Pre- and postsynaptic determinants of EPSC waveform at cerebellar climbing fiber and parallel fiber to Purkinje cell synapses. J. Neurosci. 15, 5693–5702 (1995).
Dittman, J. S. & Regehr, W. G. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J. Neurosci. 16, 1623–1633 (1996).
Selbie, L. A. & Hill, S. J. G protein-coupled-receptor cross-talk: the fine-tuning of multiple receptor-signalling pathways. Trends Pharmacol. Sci. 19, 87–93 (1998).
Jin, W., Lee, N. M., Loh, H. H. & Thayer, S. A. Opioids mobilize calcium from inositol 1,4,5-trisphosphate-sensitive stores in NG108-15 cells. J. Neurosci. 14, 1920–1929 (1994).
Hahner, L., McQuilkin, S. & Harris, R. A. Cerebellar GABAB receptors modulate function of GABAA receptors. FASEB J. 5, 2466–2472 (1991).
Tempia, F., Miniaci, M. C., Anchisi, D. & Strata, P. Postsynaptic current mediated by metabotropic glutamate receptors in cerebellar Purkinje cells. J. Neurophysiol. 80, 520–528 (1998).
Batchelor, A. M. & Garthwaite, J. Novel synaptic potentials in cerebellar Purkinje cells: probable mediation by metabotropic glutamate receptors. Neuropharmacology 32, 11–20 (1993).
Batchelor, A. M. & Garthwaite, J. Frequency detection and temporally dispersed synaptic signal association through a metabotropic glutamate receptor pathway. Nature 385, 74–77 (1997).
Bourne, H. R. & Nicoll, R. Molecular machines integrate coincident synaptic signals. Cell 72 Suppl., 65–75 (1993).
Llano, I. et al. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat. Neurosci. 3, 1256–1265 (2000).
Miyata, M. et al. Local calcium release in dendritic spines required for long-term synaptic depression. Neuron 28, 233–244 (2000).
Wang, S. S., Denk, W. & Hausser, M. Coincidence detection in single dendritic spines mediated by calcium release. Nat. Neurosci. 3, 1266–1273 (2000).
Davies, C. H., Starkey, S. J., Pozza, M. F. & Collingridge, G. L. GABA autoreceptors regulate the induction of LTP. Nature 349, 609–611 (1991).
Mott, D. D. & Lewis, D. V. Facilitation of the induction of long-term potentiation by GABAB receptors. Science 252, 1718–1720 (1991).
Komatsu, Y. GABAB receptors, monoamine receptors, and postsynaptic inositol trisphosphate-induced Ca2+ release are involved in the induction of long-term potentiation at visual cortical inhibitory synapses. J. Neurosci. 16, 6342–6352 (1996).
Rocheville, M. et al. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science 288, 154–157 (2000).
Liu, F. et al. Direct protein–protein coupling enables cross-talk between dopamine D5 and GABAA receptors. Nature 403, 274–280 (2000).
Khakh, B. S., Zhou, X., Sydes, J., Galligan, J. J. & Lester, H. A. State-dependent cross-inhibition between transmitter-gated cation channels. Nature 406, 405–410 (2000).
Acknowledgements
We thank J. Bockaert, T. Murakoshi, D. Rusakov, F. Saitow and K. Yoshioka for comments on the manuscript and Novartis Pharma (Basel, Switzerland) for the gift of CGP62349. This work was supported in part by a Grant-in-Aid 0727910 (T.Y.) from the Ministry of Education, Science, Sports and Culture of Japan, and Grant-in-Aids 96L00310 (T.Y.) and 12780603 (M.H.) from the Japan Society for Promotion of Science. S.K. is a research director of CREST, JST (Core Research for Evolutional Science and Technology, Japan Science and Technology Corporation).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hirono, M., Yoshioka, T. & Konishi, S. GABAB receptor activation enhances mGluR-mediated responses at cerebellar excitatory synapses. Nat Neurosci 4, 1207–1216 (2001). https://doi.org/10.1038/nn764
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn764