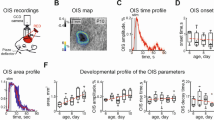
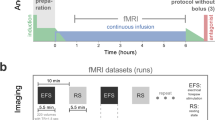
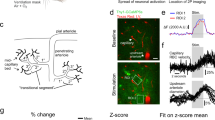
Abstract
Functional magnetic resonance imaging (fMRI) is a valuable method for probing postnatal circuit refinement and plasticity. However, its use during early development has been hindered by uncertainty as to the nature of neurovascular coupling in young individuals. Here we used somatosensory stimulation in rats to determine age-related parameters of the blood oxygenation level–dependent (BOLD) signal from its apparent inception on postnatal day 13 to adulthood. By comparing fMRI measurements with electrophysiological recordings, we determined that the regional BOLD response in these animals undergoes a systematic decline in latency and growth in amplitude over this period. We found no evidence of negative BOLD at any age. Maturation of hemodynamic responses correlated with age-dependent increases in susceptibility to inhibition of carbonic anhydrase. With knowledge of the infant BOLD response characteristics, we showed that interhemispheric and higher-order cortical stimulus responses are enhanced during the first several weeks after birth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Buxton, R.B. Introduction to Functional Magnetic Resonance Imaging: Principles and Techniques 523 (Cambridge University Press, Cambridge, 2002).
Born, P. et al. Visual activation in infants and young children studied by functional magnetic resonance imaging. Pediatr. Res. 44, 578–583 (1998).
Anderson, A.W. et al. Neonatal auditory activation detected by functional magnetic resonance imaging. Magn. Reson. Imaging 19, 1–5 (2001).
Yamada, H. et al. A rapid brain metabolic change in infants detected by fMRI. Neuroreport 8, 3775–3778 (1997).
Fulford, J. et al. Fetal brain activity and hemodynamic response to a vibroacoustic stimulus. Hum. Brain Mapp. 22, 116–121 (2004).
Hykin, J. et al. Fetal brain activity demonstrated by functional magnetic resonance imaging. Lancet 354, 645–646 (1999).
Muramoto, S. et al. Age-dependent change in metabolic response to photic stimulation of the primary visual cortex in infants: functional magnetic resonance imaging study. J. Comput. Assist. Tomogr. 26, 894–901 (2002).
Kourtzi, Z., Augath, M., Logothetis, N.K., Movshon, J.A. & Kiorpes, L. Development of visually evoked cortical activity in infant macaque monkeys studied longitudinally with fMRI. Magn. Reson. Imaging 24, 359–366 (2006).
Smith, A.J. et al. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc. Natl. Acad. Sci. USA 99, 10765–10770 (2002).
Sheth, S.A. et al. Linear and nonlinear relationships between neuronal activity, oxygen metabolism and hemodynamic responses. Neuron 42, 347–355 (2004).
Liu, Z.M., Schmidt, K.F., Sicard, K.M. & Duong, T.Q. Imaging oxygen consumption in forepaw somatosensory stimulation in rats under isoflurane anesthesia. Magn. Reson. Med. 52, 277–285 (2004).
Masamoto, K., Kim, T., Fukuda, M., Wang, P. & Kim, S.G. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb. Cortex 17, 942–950 (2007).
Logothetis, N.K., Guggenberger, H., Peled, S. & Pauls, J. Functional imaging of the monkey brain. Nat. Neurosci. 2, 555–562 (1999).
Duong, T.Q., Kim, D.S., Ugurbil, K. & Kim, S.G. Localized cerebral blood flow response at submillimeter columnar resolution. Proc. Natl. Acad. Sci. USA 98, 10904–10909 (2001).
Sanders, R.D., Patel, N., Hossain, M., Ma, D. & Maze, M. Isoflurane exerts antinociceptive and hypnotic properties at all ages in Fischer rats. Br. J. Anaesth. 95, 393–399 (2005).
Mueggler, T., Baumann, D., Rausch, M. & Rudin, M. Bicuculline-induced brain activation in mice detected by functional magnetic resonance imaging. Magn. Reson. Med. 46, 292–298 (2001).
Ramos-Cabrer, P., Weber, R., Wiedermann, D. & Hoehn, M. Continuous noninvasive monitoring of transcutaneous blood gases for a stable and persistent BOLD contrast in fMRI studies in the rat. NMR Biomed. 18, 440–446 (2005).
Dahlgren, N., Nilsson, B., Sakabe, T. & Siesjo, B.K. The effect of indomethacin on cerebral blood flow and oxygen consumption in the rat at normal and increased carbon dioxide tensions. Acta Physiol. Scand. 111, 475–485 (1981).
Kelly, P.A., Ritchie, I.M. & Arbuthnott, G.W. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole: effects upon local cerebral blood flow and glucose use in the rat. J. Cereb. Blood Flow Metab. 15, 766–773 (1995).
Brown, G.G. et al. BOLD and perfusion response to finger-thumb apposition after acetazolamide administration: differential relationship to global perfusion. J. Cereb. Blood Flow Metab. 23, 829–837 (2003).
Brion, L.P., Schwartz, J.H., Zavilowitz, B.J. & Schwartz, G.J. Micro-method for the measurement of carbonic anhydrase activity in cellular homogenates. Anal. Biochem. 175, 289–297 (1988).
Wistrand, P.J. The importance of carbonic anhydrase B and C for the unloading of CO2 by the human erythrocyte. Acta Physiol. Scand. 113, 417–426 (1981).
Swenson, E.R. & Maren, T.H. A quantitative analysis of CO2 transport at rest and during maximal exercise. Respir. Physiol. 35, 129–159 (1978).
Handwerker, D.A., Ollinger, J.M. & D'Esposito, M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage 21, 1639–1651 (2004).
Armstrong-James, M. The functional status and columnar organization of single cells responding to cutaneous stimulation in neonatal rat somatosensory cortex S1. J. Physiol. (Lond.) 246, 501–538 (1975).
Bureau, I., Shepherd, G.M. & Svoboda, K. Precise development of functional and anatomical columns in the neocortex. Neuron 42, 789–801 (2004).
Wu, C.C. & Gonzalez, M.F. Functional development of the vibrissae somatosensory system of the rat: (14C) 2-deoxyglucose metabolic mapping study. J. Comp. Neurol. 384, 323–336 (1997).
Rowan, R.A. & Maxwell, D.S. Patterns of vascular sprouting in the postnatal development of the cerebral cortex of the rat. Am. J. Anat. 160, 247–255 (1981).
Nehlig, A., Pereira de Vasconcelos, A. & Boyet, S. Postnatal changes in local cerebral blood flow measured by the quantitative autoradiographic [14C]iodoantipyrine technique in freely moving rats. J. Cereb. Blood Flow Metab. 9, 579–588 (1989).
Haydon, P.G. & Carmignoto, G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 86, 1009–1031 (2006).
Binmoller, F.J. & Muller, C.M. Postnatal development of dye-coupling among astrocytes in rat visual cortex. Glia 6, 127–137 (1992).
Kaur, C., Ling, E.A. & Wong, W.C. Development of the various glial cell types in the cerebral cortex of postnatal rats. Acta Anat. 136, 204–210 (1989).
Micheva, K.D. & Beaulieu, C. Quantitative aspects of synaptogenesis in the rat barrel field cortex with special reference to GABA circuitry. J. Comp. Neurol. 373, 340–354 (1996).
Himwich, W.A. Developmental Neurobiology (Charles C. Thomas, Springfield, 1970).
Gramsbergen, A. The development of the EEG in the rat. Dev. Psychobiol. 9, 501–515 (1976).
Ruusuvuori, E. et al. Carbonic anhydrase isoform VII acts as a molecular switch in the development of synchronous gamma-frequency firing of hippocampal CA1 pyramidal cells. J. Neurosci. 24, 2699–2707 (2004).
Vorstrup, S., Henriksen, L. & Paulson, O.B. Effect of acetazolamide on cerebral blood flow and cerebral metabolic rate for oxygen. J. Clin. Invest. 74, 1634–1639 (1984).
Apkon, M. & Boron, W.F. Extracellular and intracellular alkalinization and the constriction of rat cerebral arterioles. J. Physiol. (Lond.) 484, 743–753 (1995).
Stout, R.W., Cho, D.Y., Gaunt, S.D., Taylor, H.W. & Baker, D.G. Transcutaneous blood gas monitoring in the rat. Comp. Med. 51, 524–533 (2001).
Schuchmann, S. et al. Experimental febrile seizures are precipitated by a hyperthermia-induced respiratory alkalosis. Nat. Med. 12, 817–823 (2006).
Clancy, B., Darlington, R.B. & Finlay, B.L. Translating developmental time across mammalian species. Neuroscience 105, 7–17 (2001).
Erberich, S.G., Friedlich, P., Seri, I., Nelson, M.D., Jr. & Bluml, S. Functional MRI in neonates using neonatal head coil and MR compatible incubator. Neuroimage 20, 683–692 (2003).
Dehaene-Lambertz, G., Dehaene, S. & Hertz-Pannier, L. Functional neuroimaging of speech perception in infants. Science 298, 2013–2015 (2002).
Richter, W. & Richter, M. The shape of the fMRI BOLD response in children and adults changes systematically with age. Neuroimage 20, 1122–1131 (2003).
Rodman, H.R., Scalaidhe, S.P. & Gross, C.G. Response properties of neurons in temporal cortical visual areas of infant monkeys. J. Neurophysiol. 70, 1115–1136 (1993).
Olavarria, J.F. & Safaeian, P. Development of callosal topography in visual cortex of normal and enucleated rats. J. Comp. Neurol. 496, 495–512 (2006).
Price, D.J. et al. The development of cortical connections. Eur. J. Neurosci. 23, 910–920 (2006).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates (Elsevier Academic Press, Amsterdam, 2005).
Cox, R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173 (1996).
Acknowledgements
We thank John Marota, George Dai and Joseph Mandeville. We would also like to thank David Cory for the use of the magnet and for technical advice. The authors received generous support from the McGovern Institute for Brain Research and the Whitehead Institute for Biomedical Research, where A.J. was a Whitehead Fellow. K.K. is a member of the Nordic Center of Excellence for Research in Water Imbalance Related Disorders. Some preliminary data for this study was generated with additional support to A.J. from the Martinos Center for Biomedical Imaging at the Massachusetts General Hospital.
Author information
Authors and Affiliations
Contributions
The research was designed, carried out, analyzed and written by M.T.C. and A.J. M.C.-P. initiated the project. K.K. suggested and consulted on the carbonic anhydrase experiments. M.A.P. designed and carried out the blood carbonic anhydrase assays.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Results (PDF 3954 kb)
Rights and permissions
About this article
Cite this article
Colonnese, M., Phillips, M., Constantine-Paton, M. et al. Development of hemodynamic responses and functional connectivity in rat somatosensory cortex. Nat Neurosci 11, 72–79 (2008). https://doi.org/10.1038/nn2017
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn2017
This article is cited by
-
Arousal state transitions occlude sensory-evoked neurovascular coupling in neonatal mice
Communications Biology (2023)
-
Halftone spatial frequency domain imaging enables kilohertz high-speed label-free non-contact quantitative mapping of optical properties for strongly turbid media
Light: Science & Applications (2021)
-
Effects of urethane and isoflurane on the sensory evoked response and local blood flow in the early postnatal rat somatosensory cortex
Scientific Reports (2021)
-
Cerebrovascular development: mechanisms and experimental approaches
Cellular and Molecular Life Sciences (2021)
-
More Light on the Brain: 30 Years Later
Neuroscience and Behavioral Physiology (2019)