Abstract
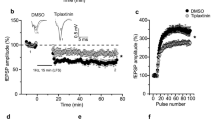
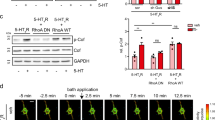
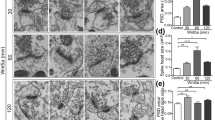
The N-methyl-D-aspartate receptor (NMDAR), brain-derived neurotrophic factor (BDNF), postsynaptic density protein 95 (PSD-95) and phosphatidylinositol 3-kinase (PI3K) have all been implicated in long-term potentiation. Here we show that these molecules are involved in a single pathway for synaptic potentiation. In visual cortical neurons in young rodents, the neurotrophin receptor TrkB is associated with PSD-95. When BDNF is applied to cultured visual cortical neurons, PSD-95–labeled synaptic puncta enlarge, and fluorescent recovery after photobleaching (FRAP) reveals increased delivery of green fluorescent protein–tagged PSD-95 to the dendrites. The recovery of fluorescence requires TrkB, signaling through PI3K and the serine-threonine kinase Akt, and an intact Golgi apparatus. Stimulation of NMDARs mimics the PSD-95 trafficking that is induced by BDNF but requires active BDNF and PI3K. Furthermore, local dendritic contact with a BDNF-coated microsphere induces PSD-95 FRAP throughout the dendrites of the stimulated neuron, suggesting that this mechanism induces rapid neuron-wide synaptic increases in PSD-95 and refinement whenever a few robust inputs activate the NMDAR-BDNF-PI3K pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kornau, H.C., Schenker, L.T., Kennedy, M.B. & Seeburg, P.H. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 269, 1737–1740 (1995).
Chen, L. et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 408, 936–943 (2000).
Beique, J.C. & Andrade, R. PSD-95 regulates synaptic transmission and plasticity in rat cerebral cortex. J. Physiol. (Lond.) 546, 859–867 (2003).
Stein, V., House, D.R., Bredt, D.S. & Nicoll, R.A. Postsynaptic density-95 mimics and occludes hippocampal long-term potentiation and enhances long-term depression. J. Neurosci. 23, 5503–5506 (2003).
Ehrlich, I. & Malinow, R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J. Neurosci. 24, 916–927 (2004).
Kim, E. & Sheng, M. PDZ domain proteins of synapses. Nat. Rev. Neurosci. 5, 771–781 (2004).
Yoshii, A., Sheng, M.H. & Constantine-Paton, M. Eye opening induces a rapid dendritic localization of PSD-95 in central visual neurons. Proc. Natl. Acad. Sci. USA 100, 1334–1339 (2003).
Lu, W. & Constantine-Paton, M. Eye opening rapidly induces synaptic potentiation and refinement. Neuron 43, 237–249 (2004).
Lessmann, V., Gottmann, K. & Malcangio, M. Neurotrophin secretion: current facts and future prospects. Prog. Neurobiol. 69, 341–374 (2003).
Poo, M.M. Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2, 24–32 (2001).
Ji, Y., Pang, P.T., Feng, L. & Lu, B. Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat. Neurosci. 8, 164–172 (2005).
McAllister, A.K., Katz, L.C. & Lo, D.C. Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 22, 295–318 (1999).
Cabelli, R.J., Hohn, A. & Shatz, C.J. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science 267, 1662–1666 (1995).
Huang, Z.J. et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98, 739–755 (1999).
Hanover, J.L., Huang, Z.J., Tonegawa, S. & Stryker, M.P. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J. Neurosci. 19, RC40.1–RC40.5 (1999).
Pang, P.T. et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science 306, 487–491 (2004).
Du, J.L. & Poo, M.M. Rapid BDNF-induced retrograde synaptic modification in a developing retinotectal system. Nature 429, 878–883 (2004).
Itami, C. et al. Brain-derived neurotrophic factor-dependent unmasking of “silent” synapses in the developing mouse barrel cortex. Proc. Natl. Acad. Sci. USA 100, 13069–13074 (2003).
Kang, H. & Schuman, E.M. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 267, 1658–1662 (1995).
Figurov, A., Pozzo-Miller, L.D., Olafsson, P., Wang, T. & Lu, B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 381, 706–709 (1996).
Kovalchuk, Y., Hanse, E., Kafitz, K.W. & Konnerth, A. Postsynaptic induction of BDNF-mediated long-term potentiation. Science 295, 1729–1734 (2002).
Suzuki, S. et al. BDNF-induced recruitment of TrkB receptor into neuronal lipid rafts: roles in synaptic modulation. J. Cell Biol. 167, 1205–1215 (2004).
Hering, H., Lin, C.C. & Sheng, M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J. Neurosci. 23, 3262–3271 (2003).
Wu, K. et al. Functional trkB neurotrophin receptors are intrinsic components of the adult brain postsynaptic density. Brain Res. Mol. Brain Res. 43, 286–290 (1996).
Sans, N. et al. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J. Neurosci. 20, 1260–1271 (2000).
Okabe, S., Urushido, T., Konno, D., Okado, H. & Sobue, K. Rapid redistribution of the postsynaptic density protein PSD-Zip45 (Homer 1c) and its differential regulation by NMDA receptors and calcium channels. J. Neurosci. 21, 9561–9571 (2001).
Man, H.Y. et al. Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron 38, 611–624 (2003).
Turner, T.J., Adams, M.E. & Dunlap, K. Calcium channels coupled to glutamate release identified by omega-Aga-IVA. Science 258, 310–313 (1992).
Tang, S.J. et al. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc. Natl. Acad. Sci. USA 99, 467–472 (2002).
Vanhaesebroeck, B. et al. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 70, 535–602 (2001).
Viard, P. et al. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat. Neurosci. 7, 939–946 (2004).
Jaworski, J., Spangler, S., Seeburg, D.P., Hoogenraad, C.C. & Sheng, M. Control of dendritic arborization by the phosphoinositide-3′-kinase-Akt-mammalian target of rapamycin pathway. J. Neurosci. 25, 11300–11312 (2005).
Kumar, V., Zhang, M.X., Swank, M.W., Kunz, J. & Wu, G.Y. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J. Neurosci. 25, 11288–11299 (2005).
Kau, T.R. et al. A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell 4, 463–476 (2003).
Ma, D. et al. Role of ER export signals in controlling surface potassium channel numbers. Science 291, 316–319 (2001).
El-Husseini Ael-D et al. Synaptic strength regulated by palmitate cycling on PSD-95. Cell 108, 849–863 (2002).
Washbourne, P. Greasing transmission: palmitoylation at the synapse. Neuron 44, 901–902 (2004).
Bhattacharyya, A. et al. Trk receptors function as rapid retrograde signal carriers in the adult nervous system. J. Neurosci. 17, 7007–7016 (1997).
Penzes, P. et al. The neuronal Rho-GEF Kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron 29, 229–242 (2001).
Kang, H. & Schuman, E.M. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science 273, 1402–1406 (1996).
Colledge, M. et al. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron 40, 595–607 (2003).
Frey, U. & Morris, R.G. Synaptic tagging and long-term potentiation. Nature 385, 533–536 (1997).
Alarcon, J.M., Barco, A. & Kandel, E.R. Capture of the late phase of long-term potentiation within and across the apical and basilar dendritic compartments of CA1 pyramidal neurons: synaptic tagging is compartment restricted. J. Neurosci. 26, 256–264 (2006).
Martin, K.C. & Kosik, K.S. Synaptic tagging—who's it? Nat. Rev. Neurosci. 3, 813–820 (2002).
Kwon, C.H. et al. Pten regulates neuronal arborization and social interaction in mice. Neuron 50, 377–388 (2006).
Chen, H.K. et al. Interaction of Akt-phosphorylated ataxin-1 with 14–3-3 mediates neurodegeneration in spinocerebellar ataxia type 1. Cell 113, 457–468 (2003).
O'Kelly, I., Butler, M.H., Zilberberg, N. & Goldstein, S.A. Forward transport. 14–3-3 binding overcomes retention in endoplasmic reticulum by dibasic signals. Cell 111, 577–588 (2002).
Michelsen, K., Yuan, H. & Schwappach, B. Hide and run. Arginine-based endoplasmic-reticulum-sorting motifs in the assembly of heteromultimeric membrane proteins. EMBO Rep. 6, 717–722 (2005).
Banker, G. & Goslin, K. Culturing Nerve Cells (MIT Press, Cambridge, Massachusetts, USA, 1991).
Parker, M.J. et al. PSD-93 regulates synaptic stability at neuronal cholinergic synapses. J. Neurosci. 24, 378–388 (2004).
Acknowledgements
We thank J. Hell for providing antibody to SAP102, D.E. Clapham and D.B. Arnold for permission to use the PSD-95–GFP construct, N. Sans and R. Wenthold for supplying the SAP102-GFP construct and E. Nedivi for comments on the manuscript. This work was supported by US National Institutes of Health grants RO1EY006039 and RO1EY014074.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Specificity of pan-TrkB antibody. (PDF 301 kb)
Supplementary Fig. 2
Whole-cell images of FRAP assay. (PDF 46666 kb)
Supplementary Fig. 3
Inhibition of Akt reduces PSD-95 Export to the Golgi. (PDF 6003 kb)
Supplementary Fig. 4
BDNF facilitates export of PSD-95 to the Golgi. (PDF 4181 kb)
Supplementary Fig. 5
Enlarged PSD-95-GFP puncta after application of a BDNF-coated bead. (PDF 602 kb)
Supplementary Fig. 6
A model for rapid, dendrite-wide, sensitization for synaptic potentiation conveyed by local NMDAR and BDNF activity-driven-PSD-95 trafficking to synapses throughout the neuron. (PDF 337 kb)
Rights and permissions
About this article
Cite this article
Yoshii, A., Constantine-Paton, M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci 10, 702–711 (2007). https://doi.org/10.1038/nn1903
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1903
This article is cited by
-
Synaptic input and Ca2+ activity in zebrafish oligodendrocyte precursor cells contribute to myelin sheath formation
Nature Neuroscience (2024)
-
eIF4E phosphorylation mediated LPS induced depressive-like behaviors via ameliorated neuroinflammation and dendritic loss
Translational Psychiatry (2023)
-
Reduced synaptic activity and dysregulated extracellular matrix pathways in midbrain neurons from Parkinson’s disease patients
npj Parkinson's Disease (2022)
-
Roles of palmitoylation in structural long-term synaptic plasticity
Molecular Brain (2021)
-
The mTOR/NF-κB Pathway Mediates Neuroinflammation and Synaptic Plasticity in Diabetic Encephalopathy
Molecular Neurobiology (2021)