Abstract
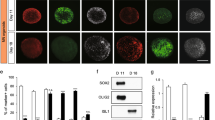
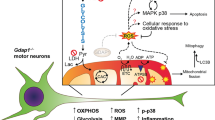
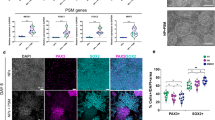
Unlike the mechanisms involved in the death of neuronal cell bodies, those causing the elimination of processes are not well understood owing to the lack of suitable experimental systems. As the neurotrophin receptor p75NTR is known to restrict the growth of neuronal processes, we engineered mouse embryonic stem (ES) cells to express an Ngfr (p75NTR) cDNA under the control of the Mapt locus (the gene encoding tau), which begins to be active when ES cell–derived progenitors start elongating processes. This caused a progressive, synchronous degeneration of all processes, and a prospective proteomic analysis showed increased levels of the sugar-binding protein galectin-1 in the p75NTR-engineered cells. Function-blocking galectin-1 antibodies prevented the degeneration of processes, and recombinant galectin-1 caused the processes of wild-type neurons to degenerate first, followed by the cell bodies. In vivo, the application of a glutamate receptor agonist, a maneuver known to upregulate p75NTR, led to an increase in the amount of galectin-1 and to the degeneration of neurons and their processes in a galectin-1–dependent fashion. Section of the sciatic nerve also rapidly upregulated levels of p75NTR and galectin-1 in terminal Schwann cells, and the elimination of nerve endings was delayed at the neuromuscular junction of mice lacking Lgals1 (the gene encoding galectin-1). These results indicate that galectin-1 actively participates in the elimination of neuronal processes after lesion, and that engineered ES cells are a useful tool for studying relevant aspects of neuronal degeneration that have been hitherto difficult to analyze.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Raff, M.C., Whitmore, A.V. & Finn, J.T. Axonal self-destruction and neurodegeneration. Science 296, 868–871 (2002).
Coleman, M. Axon degeneration mechanisms: commonality amid diversity. Nat. Rev. Neurosci. 6, 889–898 (2005).
Williams, D.W., Kondo, S., Krzyzanowska, A., Hiromi, Y. & Truman, J.W. Local caspase activity directs engulfment of dendrites during pruning. Nat. Neurosci. 9, 1234–1236 (2006).
Sagot, Y. et al. Bcl-2 overexpression prevents motoneuron cell body loss but not axonal degeneration in a mouse model of a neurodegenerative disease. J. Neurosci. 15, 7727–7733 (1995).
Whitmore, A.V., Lindsten, T., Raff, M.C. & Thompson, C.B. The proapoptotic proteins Bax and Bak are not involved in Wallerian degeneration. Cell Death Differ. 10, 260–261 (2003).
Brown, M.C., Booth, C.M., Lunn, E.R. & Perry, V.H. Delayed response to denervation in muscles of C57BL/Ola mice. Neuroscience 43, 279–283 (1991).
Chen, S. & Bisby, M.A. Impaired motor axon regeneration in the C57BL/Ola mouse. J. Comp. Neurol. 333, 449–454 (1993).
Bittner, G.D. Long-term survival of anucleate axons and its implications for nerve regeneration. Trends Neurosci. 14, 188–193 (1991).
Perry, V.H., Lunn, E.R., Brown, M.C., Cahusac, S. & Gordon, S. Evidence that the rate of Wallerian degeneration is controlled by a single autosomal dominant gene. Eur. J. Neurosci. 2, 408–413 (1990).
Mack, T.G. et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat. Neurosci. 4, 1199–1206 (2001).
Coleman, M.P. et al. An 85-kb tandem triplication in the slow Wallerian degeneration (Wlds) mouse. Proc. Natl. Acad. Sci. USA 95, 9985–9990 (1998).
Hoopfer, E.D. et al. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron 50, 883–895 (2006).
Parson, S.H., Mackintosh, C.L. & Ribchester, R.R. Elimination of motor nerve terminals in neonatal mice expressing a gene for slow Wallerian degeneration (C57Bl/Wlds). Eur. J. Neurosci. 9, 1586–1592 (1997).
Bibel, M. et al. Differentiation of mouse embryonic stem cells into a defined neuronal lineage. Nat. Neurosci. 7, 1003–1009 (2004).
Dechant, G. & Barde, Y.A. The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat. Neurosci. 5, 1131–1136 (2002).
Zagrebelsky, M. et al. The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J. Neurosci. 25, 9989–9999 (2005).
Majdan, M. et al. Transgenic mice expressing the intracellular domain of the p75 neurotrophin receptor undergo neuronal apoptosis. J. Neurosci. 17, 6988–6998 (1997).
Tucker, K.L., Meyer, M. & Barde, Y.A. Neurotrophins are required for nerve growth during development. Nat. Neurosci. 4, 29–37 (2001).
Zhai, Q. et al. Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron 39, 217–225 (2003).
Finn, J.T. et al. Evidence that Wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. J. Neurosci. 20, 1333–1341 (2000).
Perillo, N.L., Pace, K.E., Seilhamer, J.J. & Baum, L.G. Apoptosis of T cells mediated by galectin-1. Nature 378, 736–739 (1995).
Malatesta, P. et al. Neuronal or glial progeny: regional differences in radial glia fate. Neuron 37, 751–764 (2003).
Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 103, 239–252 (2000).
Maroney, A.C. et al. Cep-1347 (KT7515), a semisynthetic inhibitor of the mixed lineage kinase family. J. Biol. Chem. 276, 25302–25308 (2001).
Saporito, M.S. et al. Preservation of cholinergic activity and prevention of neuron death by CEP-1347/KT-7515 following excitotoxic injury of the nucleus basalis magnocellularis. Neuroscience 86, 461–472 (1998).
Wang, L.H., Besirli, C.G. & Johnson, E.M., Jr Mixed-lineage kinases: a target for the prevention of neurodegeneration. Annu. Rev. Pharmacol. Toxicol. 44, 451–474 (2004).
Springer, J.E., Koh, S., Tayrien, M.W. & Loy, R. Basal forebrain magnocellular neurons stain for nerve growth factor receptor: correlation with cholinergic cell bodies and effects of axotomy. J. Neurosci. Res. 17, 111–118 (1987).
Oh, J.D., Chartisathian, K., Chase, T.N. & Butcher, L.L. Overexpression of neurotrophin receptor p75 contributes to the excitotoxin-induced cholinergic neuronal death in rat basal forebrain. Brain Res. 853, 174–185 (2000).
Aggarwal, P. & Gibbs, R.B. Estrogen replacement does not prevent the loss of choline acetyltransferase–positive cells in the basal forebrain following either neurochemical or mechanical lesions. Brain Res. 882, 75–85 (2000).
Reynolds, M.L. & Woolf, C.J. Terminal Schwann cells elaborate extensive processes following denervation of the motor endplate. J. Neurocytol. 21, 50–66 (1992).
Yan, Q. & Johnson, E.M., Jr . An immunohistochemical study of the nerve growth factor receptor in developing rats. J. Neurosci. 8, 3481–3498 (1988).
Yeo, T.T. et al. Absence of p75NTR causes increased basal forebrain cholinergic neuron size, choline acetyltransferase activity, and target innervation. J. Neurosci. 17, 7594–7605 (1997).
Jaffe, A.B. & Hall, A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247–269 (2005).
Yamashita, T., Tucker, K.L. & Barde, Y.A. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron 24, 585–593 (1999).
Reichardt, L.F. Neurotrophin-regulated signalling pathways. Phil. Trans. R. Soc. Lond. B 361, 1545–1564 (2006).
Hahn, H.P. et al. Galectin-1 induces nuclear translocation of endonuclease G in caspase- and cytochrome c–independent T cell death. Cell Death Differ. 11, 1277–1286 (2004).
Puche, A.C., Poirier, F., Hair, M., Bartlett, P.F. & Key, B. Role of galectin-1 in the developing mouse olfactory system. Dev. Biol. 179, 274–287 (1996).
Poirier, F. & Robertson, E.J. Normal development of mice carrying a null mutation in the gene encoding the L14 S-type lectin. Development 119, 1229–1236 (1993).
Horie, H. et al. Galectin-1 regulates initial axonal growth in peripheral nerves after axotomy. J. Neurosci. 19, 9964–9974 (1999).
Horie, H. et al. Oxidized galectin-1 stimulates macrophages to promote axonal regeneration in peripheral nerves after axotomy. J. Neurosci. 24, 1873–1880 (2004).
McGraw, J. et al. Regulation of neuronal and glial galectin-1 expression by peripheral and central axotomy of rat primary afferent neurons. Exp. Neurol. 195, 103–114 (2005).
McGraw, J. et al. Galectin-1 in regenerating motoneurons. Eur. J. Neurosci. 20, 2872–2880 (2004).
Reichert, F., Saada, A. & Rotshenker, S. Peripheral nerve injury induces Schwann cells to express two macrophage phenotypes: phagocytosis and the galactose-specific lectin MAC-2. J. Neurosci. 14, 3231–3245 (1994).
Kato, T. et al. Galectin-1 is a component of neurofilamentous lesions in sporadic and familial amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 282, 166–172 (2001).
Unlu, M., Morgan, M.E. & Minden, J.S. Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis 18, 2071–2077 (1997).
Acknowledgements
We thank G. Dechant and A. Le Bivic for discussions, P. Barker for gifts of reagents, R. Kuhn, J. Van Oostrum and J. Voshol (Novartis) for making our collaboration possible, P.-Y. Mantel for help with the generation of ES cell lines and T. Matsumoto, V. Nikoletopoulou and V. Bischoff for comments on the experiments. This study was supported by the Swiss National Foundation.
Author information
Authors and Affiliations
Contributions
M.B. developed the ES cell–based differentiation system and generated the Mapt::Ngfr cells, and C.A. generated the Ngfr−/− ES cells. S.H. and D.M. carried out the proteomic analysis. S.B. carried out the in vivo rat experiments, and S.L. and M.R. analyzed the axotomy experiments. F.P. generated the Lgals1 mutant mice. N.P. and Y.-A.B. designed and carried out all other experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Plachta, N., Annaheim, C., Bissière, S. et al. Identification of a lectin causing the degeneration of neuronal processes using engineered embryonic stem cells. Nat Neurosci 10, 712–719 (2007). https://doi.org/10.1038/nn1897
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1897
This article is cited by
-
The emerging role of galectins in (re)myelination and its potential for developing new approaches to treat multiple sclerosis
Cellular and Molecular Life Sciences (2020)
-
GALECTIN-8 Is a Neuroprotective Factor in the Brain that Can Be Neutralized by Human Autoantibodies
Molecular Neurobiology (2019)
-
Priming increases the anti-tumor effect and therapeutic window of 177Lu-octreotate in nude mice bearing human small intestine neuroendocrine tumor GOT1
EJNMMI Research (2017)
-
Microglial carbohydrate-binding receptors for neural repair
Cell and Tissue Research (2012)