Abstract
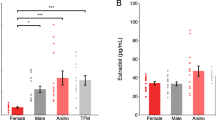
Puberty is characterized by mood swings and anxiety, which are often produced by stress. Here we show that THP (allopregnanolone), a steroid that is released as a result of stress, increases anxiety in pubertal female mice, in contrast to its anxiety-reducing effect in adults. Anxiety is regulated by GABAergic inhibition in limbic circuits. Although this inhibition is increased by THP administration before puberty and in adults, during puberty THP reduces the tonic inhibition of pyramidal cells in hippocampal region CA1, leading to increased excitability. This paradoxical effect of THP results from inhibition of α4βδ GABAA receptors. These receptors are normally expressed at very low levels, but at puberty, their expression is increased in hippocampal area CA1, where they generate outward currents. THP also decreases the outward current at recombinant α4β2δ receptors, and this effect depends on arginine 353 in the α4 subunit, a putative site for modulation by Cl−. Therefore, inhibition of α4β2δ GABAA receptors by THP provides a mechanism for the generation of anxiety at puberty.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Buchanan, C.M., Eccles, J.S. & Becker, J.B. Are adolescents the victims of raging hormones: evidence for activational effects of hormones on moods and behavior at adolescence. Psychol. Bull. 111, 62–107 (1992).
Hayward, C. & Sanborn, K. Puberty and the emergence of gender differences in psychopathology. J. Adolesc. Health 30S, 49–58 (2002).
Modesti, P.A. et al. Changes in blood pressure reactivity and 24-hour blood pressure profile occurring at puberty. Angiology 45, 443–450 (1994).
Rudolph, U. et al. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature 401, 796–800 (1999).
Majewska, M.D., Harrison, N.L., Schwartz, R.D., Barker, J.L. & Paul, S.M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232, 1004–1007 (1986).
Purdy, R.H., Morrow, A.L., Moore, P.H., Jr. & Paul, S.M. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat. Proc. Natl. Acad. Sci. USA 88, 4553–4557 (1991).
Freeman, E.W., Purdy, R.H., Coutifaris, C., Rickels, K. & Paul, S.M. Anxiolytic metabolites of progesterone: correlation with mood and performance measures following oral progesterone administration to healthy female volunteers. Neuroendocrinology 58, 478–484 (1993).
Bitran, D., Dugan, M., Renda, P., Ellis, R. & Foley, M. Anxiolytic effects of the neuroactive steroid pregnanolone (3alpha-OH-5beta-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 850, 217–224 (1999).
Chang, Y., Wang, R., Barot, S. & Weiss, D.S. Stoichiometry of a recombinant GABAA receptor. J. Neurosci. 16, 5415–5424 (1996).
Wohlfarth, K.M., Bianchi, M.T. & Macdonald, R.L. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J. Neurosci. 22, 1541–1549 (2002).
Bianchi, M.T., Haas, K.F. & Macdonald, R.L. α1 and α6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABAA receptors containing the δ subunit. Neuropharmacology 43, 492–502 (2002).
Belelli, D., Casula, A., Ling, A. & Lambert, J.J. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology 43, 651–661 (2002).
Wei, W., Zhang, N., Peng, Z., Houser, C.R. & Mody, I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J. Neurosci. 23, 10650–10661 (2003).
Stell, B.M., Brickley, S.G., Tang, C.Y., Farrant, M. & Mody, I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABA-A receptors. Proc. Natl. Acad. Sci. USA 100, 14439–14444 (2003).
Corpechot, C. et al. Brain neurosteroids during the mouse oestrous cycle. Brain Res. 766, 276–280 (1997).
Wisden, W., Laurie, D.J., Monyer, H. & Seeburg, P. The distribution of 13 GABA-A receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J. Neurosci. 12, 1040–1062 (1992).
Smith, S.S. et al. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 392, 926–929 (1998).
Smith, S.S., Ruderman, Y., Frye, C.A., Homanics, G.E. & Yuan, M. Steroid withdrawal in the mouse results in anxiogenic effects of 3α,5β-THP: a possible model of premenstrual dysphoric disorder. Psychopharmacology (Berl.) 186, 323–333 (2006).
Sundstrom-Poromaa, I. et al. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat. Neurosci. 5, 721–722 (2002).
Staley, K.J. & Mody, I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABA-A receptor-mediated postsynaptic conductance. J. Neurophysiol. 68, 197–212 (1992).
Staley, K.J. & Proctor, W.R. Modulation of mammalian dendritic GABA(A) receptor function by the kinetics of Cl- and HCO3- transport. J. Physiol. (Lond.) 519, 693–712 (1999).
Alger, B.E. & Nicoll, R.A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J. Physiol. (Lond.) 328, 125–141 (1982).
Lambert, N.A., Borroni, A.M., Grover, L.M. & Teyler, T.J. Hyperpolarizing and depolarizing GABA-A receptor-mediated dendritic inhibition in area CA1 of the rat hippocampus. J. Neurophysiol. 66, 1538–1548 (1991).
Wu, Y., Wang, W. & Richerson, G. GABA transaminase inhibition induces spontaneous and enhances depolarization-evoked GABA efflux via reversal of the GABA transporter. J. Neurosci. 21, 2630–2639 (2001).
Miyazawa, A., Fujiyoshi, Y. & Unwin, N. Structure and gating mechanism of the acetylcholine receptor pore. Nature 423, 949–955 (2003).
Kelley, S.P., Dunlop, J.I., Kirkness, E., Lambert, J.J. & Peters, J.A. A cytoplasmic region determines single-channel conductance in 5-HT3 receptors. Nature 424, 321–324 (2003).
Chen, J., Mitcheson, J.S., Lin, M. & Sanguinetti, M.C. Functional roles of charged residues in the putative voltage sensor of the HCN2 pacemaker channel. J. Biol. Chem. 275, 36465–36471 (2000).
Fadalti, M. et al. Changes of serum allopregnanolone levels in the first 2 years of life and during pubertal development. Pediatr. Res. 46, 323–327 (1999).
Caraiscos, V.B. et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha 5 subunit-containing gamma-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. USA 101, 3662–3667 (2004).
Stell, B.M. & Mody, I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J. Neurosci. 22, RC223 (2002).
Perkins, K.L. Cell-attached voltage-clamp and current-clamp recording and stimulation techniques in brain slices. J. Neurosci. Methods 154, 1–18 (2006).
Belelli, D., Peden, D.R., Rosahl, T.W., Wafford, K. & Lambert, J.J. Extrasynaptic GABA-A receptors for thalamocortical neurons: A molecular target for hypnotics. J. Neurosci. 25, 11513–11520 (2005).
Ulrich, D. & Huguenard, J.R. Nucleus-specific chloride homeostasis in rat thalamus. J. Neurosci. 17, 2348–2354 (1997).
Girdler, S.S., Beth Mechlin, M., Light, K.C. & Morrow, A.L. Ethnic differences in allopregnanolone concentrations in women during rest and following mental stress. Psychophysiology 43, 331–336 (2006).
McEwen, B.S. Stressed or stressed out: what is the difference? J. Psychiatry Neurosci. 30, 315–318 (2005).
Lundgren, P., Stromberg, J., Backstrom, T. & Wang, M. Allopregnanolone-stimulated GABA-mediated chloride ion flux is inhibited by 3beta-hydroxy-5alpha-pregnan-20-one (isoallopregnanolone). Brain Res. 982, 45–53 (2003).
Ramsey, I.S., Moran, M.M., Chong, J.A. & Clapham, D.E. A voltage-gated proton-selective channel lacking the pore domain. Nature 440, 1213–1216 (2006).
Olsen, R.W. & Snowman, A. Chloride-dependent enhancement by barbiturates of gamma-aminobutyric acid receptor binding. J. Neurosci. 2, 1812–1823 (1982).
Turner, J.H. & Raymond, J.R. Interaction of calmodulin with the serotoin 5-hydroxytryptamine2A receptor. A putative regulator of G protein coupling and receptor phosphorylation by protein kinase C. J. Biol. Chem. 280, 30741–30750 (2005).
Haas, K.F. & Macdonald, R.L. GABAA receptor subunit gamma2 and delta subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J. Physiol. (Lond.) 514, 27–45 (1999).
Chen, X. et al. Inhibition of a background potassium channel by Gq protein alpha-subunits. Proc. Natl. Acad. Sci. USA 103, 3422–3427 (2006).
Prescott, S.A. & de Koninck, Y. Gain control of firing rate by shunting inhibition: roles of synaptic noise and dendritic saturation. Proc. Natl. Acad. Sci. USA 100, 2076–2081 (2003).
Bai, D. et al. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by y-aminobutyric acid(A) receptors in hippocampal neurons. Mol. Pharmacol. 59, 814–824 (2000).
Maguire, J.L., Stell, B.M., Rafizadeh, M. & Mody, I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat. Neurosci. 8, 797–804 (2005).
Romeo, R.D. Neuroendocrine and behavioral development during puberty: a tale of two axes. Vitam. Horm. 71, 1–25 (2005).
Agis-Balboa, R.C. et al. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc. Natl. Acad. Sci. USA 103, 14602–14607 (2006).
Mele, P. et al. Increased expression of the gene for the Y1 receptor of the neuropeptde Y in the amygdala and paraventricular nucleus of Y1R/LacZ transgenic mice in response to restraint stress. J. Neurochem. 89, 1471–1478 (2004).
Freeman, E.W., Frye, C.A., Rickels, K., Martin, P.A. & Smith, S.S. Allopregnanolone levels and symptom improvement in severe premenstrual syndrome. J. Clin. Psychopharmacol. 22, 516–520 (2002).
Schmidt, P.J., Nieman, L.K., Danaceau, M.A., Adams, L.F. & Rubinow, D.R. Differential behavioral effects of gonadal steroids in women with premenstrual syndrome. N. Engl. J. Med. 338, 209–216 (1998).
Andreen, L. et al. Relationship between allopregnanolone and negative mood in postmenopausal women taking sequential hormone replacement therapy with vaginal progesterone. Psychoneuroendocrinology 30, 212–224 (2004).
Acknowledgements
We thank C.A. Frye for performing steroid assays on hippocampal tissue, W. Sieghart for supplying the δ antibody, G. Homanics for supplying and genotyping the δ−/− mice, K. Perkins and D.H. Smith for discussions, and C. McBain and J. Celentano for reading the manuscript. The work in this study was supported by grants from the US National Institutes of Health (to S.S.S., C.A. and K.W.) and from the US National Institute of Alcoholism and Alcohol Abuse (to S.S.S.).
Author information
Authors and Affiliations
Contributions
H.S. (slice physiology) and Q.H.G. (recombinant receptors) performed the electrophysiology experiments, C.A. performed the immunohistochemical experiments, M.Y. conducted the western blot and molecular studies, Y.R. conducted the behavioral experiments, M.D. did the transfections, K.W. supervised the molecular studies and contributed to the writing and S.S.S. supervised the study, performed the mutagenesis studies and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
GABA concentration-response relationships and THP administration. (PDF 70 kb)
Supplementary Fig. 2
THP inhibition of outward current is dependent upon GABA concentration. (PDF 72 kb)
Supplementary Fig. 3
Pharmacological changes in the tonic inhibitory current of CA1 hippocampus are consistent with increased expression of α4βδ receptors after the onset of puberty. (PDF 128 kb)
Supplementary Fig. 4
THP increases the input resistance of hippocampal pyramidal cells at the onset of puberty. (PDF 30 kb)
Supplementary Table 1
ANOVA – F Tables – recombinant receptor experiments. (PDF 50 kb)
Supplementary Table 2
Mutations. (PDF 36 kb)
Supplementary Table 3
Tukey's test—planned post-hoc comparisons. Mutations, 1 μM GABA. (PDF 48 kb)
Supplementary Table 4
Tukey's test—planned post-hoc comparisons. Mutations, 10 μM GABA. (PDF 41 kb)
Supplementary Table 5
Western blot. (PDF 40 kb)
Supplementary Table 6
Slice pharmacology. (PDF 35 kb)
Supplementary Table 7
Tonic current. (PDF 32 kb)
Supplementary Table 8
Slice physiology. (PDF 32 kb)
Supplementary Table 9
Tukey's post-hoc comparisons. (PDF 43 kb)
Supplementary Table 10
Neuronal excitability. (PDF 35 kb)
Supplementary Table 11
Cell-attached spiking, Tukey's test—planned post-hoc comparisons. (PDF 42 kb)
Supplementary Table 12
Two-way ANOVA. (PDF 35 kb)
Supplementary Table 13
Holm-Sidak post-hoc comparison. (PDF 52 kb)
Supplementary Table 14
ANOVA, elevated plus maze. (PDF 33 kb)
Supplementary Table 15
Least significant difference planned post-hoc comparisons. (PDF 55 kb)
Rights and permissions
About this article
Cite this article
Shen, H., Gong, Q., Aoki, C. et al. Reversal of neurosteroid effects at α4β2δ GABAA receptors triggers anxiety at puberty. Nat Neurosci 10, 469–477 (2007). https://doi.org/10.1038/nn1868
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1868
This article is cited by
-
Cyclin D1—Cdk4 regulates neuronal activity through phosphorylation of GABAA receptors
Cellular and Molecular Life Sciences (2023)
-
Thalamocortical bistable switch as a theoretical model of fibromyalgia pathogenesis inferred from a literature survey
Journal of Computational Neuroscience (2022)
-
Activity-based anorexia animal model: a review of the main neurobiological findings
Journal of Eating Disorders (2021)
-
The effect of reproductive hormones on women’s daily smoking across the menstrual cycle
Biology of Sex Differences (2021)
-
Preventing adolescent synaptic pruning in mouse prelimbic cortex via local knockdown of α4βδ GABAA receptors increases anxiety response in adulthood
Scientific Reports (2021)