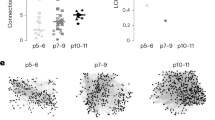
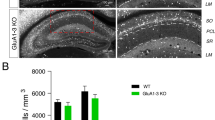
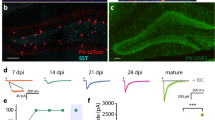
Abstract
Feedforward inhibitory GABAergic transmission is critical for mature cortical circuit function; in the neonate, however, GABA is depolarizing and believed to have a different role. Here we show that the GABAA receptor–mediated conductance is depolarizing in excitatory (stellate) cells in neonatal (postnatal day [P]3–5) layer IV barrel cortex, but GABAergic transmission at this age is not engaged by thalamocortical input in the feedforward circuit and has no detectable circuit function. However, recruitment occurs at P6–7 as a result of coordinated increases in thalamic drive to fast-spiking interneurons, fast-spiking interneuron–stellate cell connectivity and hyperpolarization of the GABAA receptor–mediated response. Thus, GABAergic circuits are not engaged by thalamocortical input in the neonate, but are poised for a remarkably coordinated development of feedforward inhibition at the end of the first postnatal week, which has profound effects on circuit function at this critical time in development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Woolsey, T.A. & Van der Loos, H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 17, 205–242 (1970).
Fox, K. Anatomical pathways and molecular mechanisms for plasticity in the barrel cortex. Neuroscience 111, 799–814 (2002).
Feldman, D.E., Nicoll, R.A. & Malenka, R.C. Synaptic plasticity at thalamocortical synapses in developing rat somatosensory cortex: LTP, LTD, and silent synapses. J. Neurobiol. 41, 92–101 (1999).
Foeller, E. & Feldman, D.E. Synaptic basis for developmental plasticity in somatosensory cortex. Curr. Opin. Neurobiol. 14, 89–95 (2004).
Castro-Alamancos, M.A. & Connors, B.W. Thalamocortical synapses. Prog. Neurobiol. 51, 581–606 (1997).
Daw, M.I., Bannister, N.V. & Isaac, J.T. Rapid, activity-dependent plasticity in timing precision in neonatal barrel cortex. J. Neurosci. 26, 4178–4187 (2006).
Bannister, N.J. et al. Developmental changes in AMPA and kainate receptor–mediated quantal transmission at thalamocortical synapses in the barrel cortex. J. Neurosci. 25, 5259–5271 (2005).
Isaac, J.T., Crair, M.C., Nicoll, R.A. & Malenka, R.C. Silent synapses during development of thalamocortical inputs. Neuron 18, 269–280 (1997).
Crair, M.C. & Malenka, R.C. A critical period for long-term potentiation at thalamocortical synapses. Nature 375, 325–328 (1995).
Kidd, F.L. & Isaac, J.T. Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature 400, 569–573 (1999).
Barth, A.L. & Malenka, R.C. NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat. Neurosci. 4, 235–236 (2001).
Fox, K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J. Neurosci. 12, 1826–1838 (1992).
Feldmeyer, D., Egger, V., Lubke, J. & Sakmann, B. Reliable synaptic connections between pairs of excitatory layer 4 neurones within a single 'barrel' of developing rat somatosensory cortex. J. Physiol. (Lond.) 521, 169–190 (1999).
Egger, V., Feldmeyer, D. & Sakmann, B. Coincidence detection and changes of synaptic efficacy in spiny stellate neurons in rat barrel cortex. Nat. Neurosci. 2, 1098–1105 (1999).
Arabzadeh, E., Panzeri, S. & Diamond, M.E. Whisker vibration information carried by rat barrel cortex neurons. J. Neurosci. 24, 6011–6020 (2004).
Petersen, R.S., Panzeri, S. & Diamond, M.E. Population coding in somatosensory cortex. Curr. Opin. Neurobiol. 12, 441–447 (2002).
Bruno, R.M. & Sakmann, B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science 312, 1622–1627 (2006).
Owens, D.F. & Kriegstein, A.R. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 3, 715–727 (2002).
Ben-Ari, Y. Excitatory actions of GABA during development: the nature of the nurture. Nat. Rev. Neurosci. 3, 728–739 (2002).
Gabernet, L., Jadhav, S.P., Feldman, D.E., Carandini, M. & Scanziani, M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron 48, 315–327 (2005).
Mountcastle, V.B. & Powell, T.P. Neural mechanisms subserving cutaneous sensibility, with special reference to the role of afferent inhibition in sensory perception and discrimination. Bull. Johns Hopkins Hosp. 105, 201–232 (1959).
Wehr, M. & Zador, A.M. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426, 442–446 (2003).
Ben-Ari, Y., Cherubini, E., Corradetti, R. & Gaiarsa, J.L. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol. (Lond.) 416, 303–325 (1989).
Leinekugel, X., Medina, I., Khalilov, I., Ben-Ari, Y. & Khazipov, R. Ca2+ oscillations mediated by the synergistic excitatory actions of GABAA and NMDA receptors in the neonatal hippocampus. Neuron 18, 243–255 (1997).
Akerman, C.J. & Cline, H.T. Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J. Neurosci. 26, 5117–5130 (2006).
Agmon, A. & Connors, B.W. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience 41, 365–379 (1991).
Agmon, A., Hollrigel, G. & O'Dowd, D.K. Functional GABAergic synaptic connection in neonatal mouse barrel cortex. J. Neurosci. 16, 4684–4695 (1996).
Beierlein, M., Gibson, J.R. & Connors, B.W. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J. Neurophysiol. 90, 2987–3000 (2003).
Keller, A. & White, E.L. Synaptic organization of GABAergic neurons in the mouse SmI cortex. J. Comp. Neurol. 262, 1–12 (1987).
Sun, Q.Q., Huguenard, J.R. & Prince, D.A. Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J. Neurosci. 26, 1219–1230 (2006).
Staiger, J.F., Zilles, K. & Freund, T.F. Distribution of GABAergic elements postsynaptic to ventroposteromedial thalamic projections in layer IV of rat barrel cortex. Eur. J. Neurosci. 8, 2273–2285 (1996).
Gibson, J.R., Beierlein, M. & Connors, B.W. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402, 75–79 (1999).
Beierlein, M., Fall, C.P., Rinzel, J. & Yuste, R. Thalamocortical bursts trigger recurrent activity in neocortical networks: layer 4 as a frequency-dependent gate. J. Neurosci. 22, 9885–9894 (2002).
Reyes, A. et al. Target-cell–specific facilitation and depression in neocortical circuits. Nat. Neurosci. 1, 279–285 (1998).
Naundorf, B., Wolf, F. & Volgushev, M. Unique features of action potential initiation in cortical neurons. Nature 440, 1060–1063 (2006).
Porter, J.T., Johnson, C.K. & Agmon, A. Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. J. Neurosci. 21, 2699–2710 (2001).
Ma, Y., Hu, H., Berrebi, A.S., Mathers, P.H. & Agmon, A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J. Neurosci. 26, 5069–5082 (2006).
Oliva, A.A., Jr., Jiang, M., Lam, T., Smith, K.L. & Swann, J.W. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J. Neurosci. 20, 3354–3368 (2000).
Agmon, A. & O'Dowd, D.K. NMDA receptor–mediated currents are prominent in the thalamocortical synaptic response before maturation of inhibition. J. Neurophysiol. 68, 345–349 (1992).
Luhmann, H.J. & Prince, D.A. Postnatal maturation of the GABAergic system in rat neocortex. J. Neurophysiol. 65, 247–263 (1991).
Kim, H.G., Fox, K. & Connors, B.W. Properties of excitatory synaptic events in neurons of primary somatosensory cortex of neonatal rats. Cereb. Cortex 5, 148–157 (1995).
Burgard, E.C. & Hablitz, J.J. Developmental changes in NMDA and non-NMDA receptor–mediated synaptic potentials in rat neocortex. J. Neurophysiol. 69, 230–240 (1993).
Kriegstein, A.R., Suppes, T. & Prince, D.A. Cellular and synaptic physiology and epileptogenesis of developing rat neocortical neurons in vitro. Brain Res. 431, 161–171 (1987).
Wells, J.E., Porter, J.T. & Agmon, A. GABAergic inhibition suppresses paroxysmal network activity in the neonatal rodent hippocampus and neocortex. J. Neurosci. 20, 8822–8830 (2000).
Micheva, K.D. & Beaulieu, C. Quantitative aspects of synaptogenesis in the rat barrel field cortex with special reference to GABA circuitry. J. Comp. Neurol. 373, 340–354 (1996).
Ge, S. et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439, 589–593 (2006).
Feldman, D.E., Nicoll, R.A., Malenka, R.C. & Isaac, J.T. Long-term depression at thalamocortical synapses in developing rat somatosensory cortex. Neuron 21, 347–357 (1998).
Acknowledgements
We are grateful to K. Roche, K. Pelkey and J. Diamond for comments on the manuscript, and to C. McBain for comments on the work and providing access to the GIN mice. We thank Z. Molnar for the use of his drawing of the thalamocortical slice. This work was supported by the Wellcome Trust and the National Institute of Neurological Disorders and Stroke Intramural Program.
Author information
Authors and Affiliations
Contributions
M.I.D. conducted, designed and analyzed the majority of experiments. M.C.A. conducted some of the electrophysiolgy experiments, performed all the imaging and contributed to experimental design and analysis. J.T.R.I. supervised the project, wrote the manuscript and contributed to the experimental design and analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Non-FS interneurons are distinct from fast-spiking interneurons and are inhibitory. (PDF 97 kb)
Supplementary Fig. 2
Non-FS interneurons receive a weak thalamic input compared with simultaneously recorded stellate cells or with fast-spiking interneurons at the same developmental stage (all at P7–9). (PDF 81 kb)
Supplementary Fig. 3
Electrophysiological properties of fast-spiking interneurons and stellate cells at different ages between P3 and P9. (PDF 108 kb)
Rights and permissions
About this article
Cite this article
Daw, M., Ashby, M. & Isaac, J. Coordinated developmental recruitment of latent fast spiking interneurons in layer IV barrel cortex. Nat Neurosci 10, 453–461 (2007). https://doi.org/10.1038/nn1866
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1866
This article is cited by
-
Developmental delays in cortical auditory temporal processing in a mouse model of Fragile X syndrome
Journal of Neurodevelopmental Disorders (2023)
-
Fast-spiking parvalbumin-positive interneurons in brain physiology and Alzheimer’s disease
Molecular Psychiatry (2023)
-
Step by step: cells with multiple functions in cortical circuit assembly
Nature Reviews Neuroscience (2022)
-
Myelination of parvalbumin interneurons shapes the function of cortical sensory inhibitory circuits
Nature Communications (2020)
-
Cellular and synaptic phenotypes lead to disrupted information processing in Fmr1-KO mouse layer 4 barrel cortex
Nature Communications (2019)