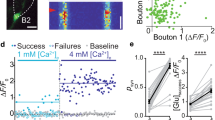
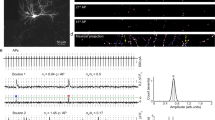
Abstract
Vesicular release of neurotransmitter is the universal output signal of neurons in the brain. It is generally believed that fast transmitter release is restricted to nerve terminals that contact postsynaptic cells in the gray matter. Here we show in the rat brain that the neurotransmitter glutamate is also released at discrete sites along axons in white matter in the absence of neurons and nerve terminals. The propagation of single action potentials along axons leads to rapid vesicular release of glutamate, which is detected by ionotropic glutamate receptors on local oligodendrocyte precursor cells. Axonal release of glutamate is reliable, involves highly localized calcium microdomain signaling and is strongly calcium cooperative, similar to vesicle fusion at synapses. This axonal transmitter release represents a widespread mechanism for high-fidelity, activity-dependent signaling at the axon-glia interface in white matter.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Jahn, R., Lang, T. & Sudhof, T.C. Membrane fusion. Cell 112, 519–533 (2003).
Rosenmund, C., Rettig, J. & Brose, N. Molecular mechanisms of active zone function. Curr. Opin. Neurobiol. 13, 509–519 (2003).
Matsui, K. & Jahr, C.E. Ectopic release of synaptic vesicles. Neuron 40, 1173–1183 (2003).
Coggan, J.S. et al. Evidence for ectopic neurotransmission at a neuronal synapse. Science 309, 446–451 (2005).
Barres, B.A., Chun, L.L. & Corey, D.P. Ion channels in vertebrate glia. Annu. Rev. Neurosci. 13, 441–474 (1990).
Steinhauser, C. & Gallo, V. News on glutamate receptors in glial cells. Trends Neurosci. 19, 339–345 (1996).
Berger, T., Schnitzer, J. & Kettenmann, H. Developmental changes in the membrane current pattern, K+ buffer capacity, and morphology of glial cells in the corpus callosum slice. J. Neurosci. 11, 3008–3024 (1991).
Baumann, N. & Pham-Dinh, D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 81, 871–927 (2001).
Sommer, I. & Schachner, M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev. Biol. 83, 311–327 (1981).
Jacobson, S. & Trojanowski, J.Q. The cells of origin of the corpus callosum in rat, cat and rhesus monkey. Brain Res. 74, 149–155 (1974).
Hume, R.I., Dingledine, R. & Heinemann, S.F. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science 253, 1028–1031 (1991).
Bowie, D. & Mayer, M.L. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15, 453–462 (1995).
Washburn, M.S. & Dingledine, R. Block of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by polyamines and polyamine toxins. J. Pharmacol. Exp. Ther. 278, 669–678 (1996).
Clements, J.D. Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosci. 19, 163–171 (1996).
Liu, G., Choi, S. & Tsien, R.W. Variability of neurotransmitter concentration and nonsaturation of postsynaptic AMPA receptors at synapses in hippocampal cultures and slices. Neuron 22, 395–409 (1999).
Raastad, M., Storm, J.F. & Andersen, P. Putative single quantum and single fibre excitatory postsynaptic currents show similar amplitude range and variability in rat hippocampal slices. Eur. J. Neurosci. 4, 113–117 (1992).
Garner, C.C., Zhai, R.G., Gundelfinger, E.D. & Ziv, N.E. Molecular mechanisms of CNS synaptogenesis. Trends Neurosci. 25, 243–250 (2002).
Taschenberger, H., Scheuss, V. & Neher, E. Release kinetics, quantal parameters and their modulation during short-term depression at a developing synapse in the rat CNS. J. Physiol. (Lond.) 568, 513–537 (2005).
Augustine, G.J. How does calcium trigger neurotransmitter release? Curr. Opin. Neurobiol. 11, 320–326 (2001).
Dietrich, D. et al. Functional specialization of presynaptic Cav2.3 Ca2+ channels. Neuron 39, 483–496 (2003).
Schneggenburger, R. & Neher, E. Presynaptic calcium and control of vesicle fusion. Curr. Opin. Neurobiol. 15, 266–274 (2005).
Hille, B. Ionic Channels of Excitable Membranes (Sinauer, Sunderland, Massachusetts, USA, 1992).
Neher, E. Usefulness and limitations of linear approximations to the understanding of Ca++ signals. Cell Calcium 24, 345–357 (1998).
Mintz, I.M., Sabatini, B.L. & Regehr, W.G. Calcium control of transmitter release at a cerebellar synapse. Neuron 15, 675–688 (1995).
Barrett, E.F. & Stevens, C.F. The kinetics of transmitter release at the frog neuromuscular junction. J. Physiol. (Lond.) 227, 691–708 (1972).
Van der Kloot, W. Estimating the timing of quantal releases during end-plate currents at the frog neuromuscular junction. J. Physiol. (Lond.) 402, 595–603 (1988).
Meinrenken, C.J., Borst, J.G. & Sakmann, B. Local routes revisited: the space and time dependence of the Ca2+ signal for phasic transmitter release at the rat calyx of Held. J. Physiol. (Lond.) 547, 665–689 (2003).
Simon, S.M. & Llinas, R.R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys. J. 48, 485–498 (1985).
Augustine, G.J., Santamaria, F. & Tanaka, K. Local calcium signaling in neurons. Neuron 40, 331–346 (2003).
Naraghi, M. T-jump study of calcium binding kinetics of calcium chelators. Cell Calcium 22, 255–268 (1997).
Sabatini, B.L. & Regehr, W.G. Optical measurement of presynaptic calcium currents. Biophys. J. 74, 1549–1563 (1998).
Pyle, J.L., Kavalali, E.T., Choi, S. & Tsien, R.W. Visualization of synaptic activity in hippocampal slices with FM1-43 enabled by fluorescence quenching. Neuron 24, 803–808 (1999).
Stanton, P.K. et al. Long-term depression of presynaptic release from the readily releasable vesicle pool induced by NMDA receptor-dependent retrograde nitric oxide. J. Neurosci. 23, 5936–5944 (2003).
Spacek, J. & Harris, K.M. Trans-endocytosis via spinules in adult rat hippocampus. J. Neurosci. 24, 4233–4241 (2004).
Sturrock, R.R. Myelination of the mouse corpus callosum. Neuropathol. Appl. Neurobiol. 6, 415–420 (1980).
Bjartmar, C., Hildebrand, C. & Loinder, K. Morphological heterogeneity of rat oligodendrocytes: electron microscopic studies on serial sections. Glia 11, 235–244 (1994).
Trapp, B.D., Nishiyama, A., Cheng, D. & Macklin, W. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J. Cell Biol. 137, 459–468 (1997).
Riederer, B.M., Berbel, P. & Innocenti, G.M. Neurons in the corpus callosum of the cat during postnatal development. Eur. J. Neurosci. 19, 2039–2046 (2004).
Temple, S. & Raff, M.C. Clonal analysis of oligodendrocyte development in culture: evidence for a developmental clock that counts cell divisions. Cell 44, 773–779 (1986).
Carleton, A., Petreanu, L.T., Lansford, R., Alvarez-Buylla, A. & Lledo, P.M. Becoming a new neuron in the adult olfactory bulb. Nat. Neurosci. 6, 507–518 (2003).
Ge, S. et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439, 589–593 (2006).
Zhen, M. & Jin, Y. Presynaptic terminal differentiation: transport and assembly. Curr. Opin. Neurobiol. 14, 280–287 (2004).
Li, S., Mealing, G.A., Morley, P. & Stys, P.K. Novel injury mechanism in anoxia and trauma of spinal cord white matter: glutamate release via reverse Na+-dependent glutamate transport. J. Neurosci. 19, RC16 (1999).
Kriegler, S. & Chiu, S.Y. Calcium signaling of glial cells along mammalian axons. J. Neurosci. 13, 4229–4245 (1993).
Fields, R.D. & Burnstock, G. Purinergic signalling in neuron-glia interactions. Nat. Rev. Neurosci. 7, 423–436 (2006).
Barres, B.A. & Raff, M.C. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361, 258–260 (1993).
Demerens, C. et al. Induction of myelination in the central nervous system by electrical activity. Proc. Natl. Acad. Sci. USA 93, 9887–9892 (1996).
Karadottir, R., Cavelier, P., Bergersen, L.H. & Attwell, D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438, 1162–1166 (2005).
Chittajallu, R., Aguirre, A. & Gallo, V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J. Physiol. (Lond.) 561, 109–122 (2004).
Stricker, C. & Redman, S.J. Quantal analysis based on density estimation. J. Neurosci. Methods 130, 159–171 (2003).
Acknowledgements
We thank S. Schoch for discussions and comments on the manuscript and for sharing reagents; D. Thal and J. Bedorf for help with electron microscopy; R. Buettner and D. Thal for sharing equipment; B. Stallcup and B. Zalc for providing antibodies. This study was supported by Deutsche Forschungsgemeinschaft (SFB TR3, DI 853/2) and University Clinic Bonn grants (BONFOR). We thank P. Stausberg and K. Neitzert for technical assistance.
Author information
Authors and Affiliations
Contributions
M.K. performed patch-clamp recordings; E.C.-Z. and D.D. performed and designed electron microscopy investigations; and D.D. carried out Ca2+- and FM1-43 imaging experiments, extracellular recordings, immunohistochemistry and three-dimensional reconstructions. D.D. and M.K. designed experiments, analyzed data and prepared figures. D.D., M.K. and E.C.-Z. wrote the manuscript. D.D. provided financial support.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Estimation of the size of the readily releasable pool and of the vesicular release probability. (PDF 145 kb)
Supplementary Fig. 2
A fit of a sum of convolved miniature AGC distributions to the distribution of minimal stimulation amplitudes yields probabilities consistent with a simple binomial process. (PDF 139 kb)
Supplementary Fig. 3
Calcium microdomains trigger axonal transmitter release. Estimation of the distance between calcium channels and synaptic vesicles. (PDF 108 kb)
Supplementary Fig. 4
Typical current patterns of callosal OPCs and pyramidal neurons recorded in whole cell voltage clamp mode. (PDF 100 kb)
Rights and permissions
About this article
Cite this article
Kukley, M., Capetillo-Zarate, E. & Dietrich, D. Vesicular glutamate release from axons in white matter. Nat Neurosci 10, 311–320 (2007). https://doi.org/10.1038/nn1850
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1850
This article is cited by
-
Oligodendrocyte–axon metabolic coupling is mediated by extracellular K+ and maintains axonal health
Nature Neuroscience (2024)
-
Norepinephrine regulates calcium signals and fate of oligodendrocyte precursor cells in the mouse cerebral cortex
Nature Communications (2023)
-
Characterization of a new mouse line triggering transient oligodendrocyte progenitor depletion
Scientific Reports (2023)
-
Intracranial electrophysiological and structural basis of BOLD functional connectivity in human brain white matter
Nature Communications (2023)
-
Axo-glial interactions between midbrain dopamine neurons and oligodendrocyte lineage cells in the anterior corpus callosum
Brain Structure and Function (2023)