Abstract
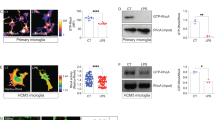
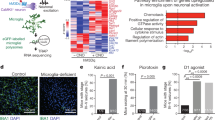
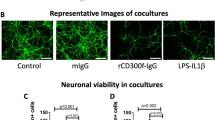
Microglia are primary immune sentinels of the CNS. Following injury, these cells migrate or extend processes toward sites of tissue damage. CNS injury is accompanied by release of nucleotides, serving as signals for microglial activation or chemotaxis. Microglia express several purinoceptors, including a Gi-coupled subtype that has been implicated in ATP- and ADP-mediated migration in vitro. Here we show that microglia from mice lacking Gi-coupled P2Y12 receptors exhibit normal baseline motility but are unable to polarize, migrate or extend processes toward nucleotides in vitro or in vivo. Microglia in P2ry12−/− mice show significantly diminished directional branch extension toward sites of cortical damage in the living mouse. Moreover, P2Y12 expression is robust in the 'resting' state, but dramatically reduced after microglial activation. These results imply that P2Y12 is a primary site at which nucleotides act to induce microglial chemotaxis at early stages of the response to local CNS injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758 (2005).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
Stence, N., Waite, M. & Dailey, M.E. Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia 33, 256–266 (2001).
Chao, C.C., Hu, S., Molitor, T.W., Shaskan, E.G. & Peterson, P.K. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J. Immunol. 149, 2736–2741 (1992).
Kreutzberg, G.W. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 19, 312–318 (1996).
Inoue, K. Microglial activation by purines and pyrimidines. Glia 40, 156–163 (2002).
Sasaki, Y. et al. Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia 44, 242–250 (2003).
Coull, J.A. et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021 (2005).
Hanisch, U.-K. in Microglia in the Regenerating and Degenerating Central Nervous System (ed. Streit, W.J.) 79–124 (Springer, New York, 2002).
Honda, S. et al. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J. Neurosci. 21, 1975–1982 (2001).
Burnstock, G. in P2 Purinoceptors: Localization, Funciton, and Transduction Mechanisms (eds. Chadwick, D. & Goode, J.) 1–34 (Wiley, New York, 1996).
Hollopeter, G. et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409, 202–207 (2001).
Zhang, F.L. et al. ADP is the cognate ligand for the orphan G protein-coupled receptor SP1999. J. Biol. Chem. 276, 8608–8615 (2001).
Andre, P. et al. P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J. Clin. Invest. 112, 398–406 (2003).
Foster, C.J. et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J. Clin. Invest. 107, 1591–1598 (2001).
Brass, S. Cardiovascular biology. Small cells, big issues. Nature 409, 145–147 (2001).
Herbert, J.M. & Savi, P. P2Y12, a new platelet ADP receptor, target of clopidogrel. Semin. Vasc. Med. 3, 113–122 (2003).
Jung, S. et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Streit, W.J. in Microglia in the Regenerating and Degenerating Central Nervous System (ed. Streit, W.J.) 1–14 (Springer, New York, 2002).
Streit, W.J., Graeber, M.B. & Kreutzberg, G.W. Functional plasticity of microglia: a review. Glia 1, 301–307 (1988).
Lee, J.C. et al. Accelerated cerebral ischemic injury by activated macrophages/microglia after lipopolysaccharide microinjection into rat corpus callosum. Glia 50, 168–181 (2005).
Moller, T., Kann, O., Verkhratsky, A. & Kettenmann, H. Activation of mouse microglial cells affects P2 receptor signaling. Brain Res. 853, 49–59 (2000).
Hardy, A.R. et al. P2Y1 and P2Y12 receptors for ADP desensitize by distinct kinase-dependent mechanisms. Blood 105, 3552–3560 (2005).
Salimi, K. & Humpel, C. Down-regulation of complement receptor 3 and major histocompatibility complex I and II antigen-like immunoreactivity accompanies ramification in isolated rat microglia. Brain Res. 946, 283–289 (2002).
Kurpius, D., Wilson, N., Fuller, L., Hoffman, A. & Dailey, M.E. Early activation, motility, and homing of neonatal microglia to injured neurons does not require protein synthesis. Glia 54, 58–70 (2006).
Cook, S.P. & McCleskey, E.W. Cell damage excites nociceptors through release of cytosolic ATP. Pain 95, 41–47 (2002).
Tsuda, M. et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424, 778–783 (2003).
Chakfe, Y. et al. ADP and AMP induce interleukin-1beta release from microglial cells through activation of ATP-primed P2X7 receptor channels. J. Neurosci. 22, 3061–3069 (2002).
Suzuki, T. et al. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J. Neurosci. 24, 1–7 (2004).
Communi, D. et al. Identification of a novel human ADP receptor coupled to G(i). J. Biol. Chem. 276, 41479–41485 (2001).
Giulian, D. & Baker, T.J. Characterization of ameboid microglia isolated from developing mammalian brain. J. Neurosci. 6, 2163–2178 (1986).
Allen, W.E., Zicha, D., Ridley, A.J. & Jones, G.E. A role for Cdc42 in macrophage chemotaxis. J. Cell Biol. 141, 1147–1157 (1998).
Caterina, M.J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997).
Luecke, H.F. & Yamamoto, K.R. The glucocorticoid receptor blocks P-TEFb recruitment by NFkappaB to effect promoter-specific transcriptional repression. Genes Dev. 19, 1116–1127 (2005).
Acknowledgements
We are grateful to L. Fuller, H. Morisaki, J. Braz and A. So for experimental advice and assistance, and to D. Littman (Skirball Institute, New York University) for providing Cx3cr1+/GFP mice. This work was supported by predoctoral fellowships from the US National Institute of General Medical Sciences (GM56847 for S.H.) and the American Heart Association (G.H.) and by research grants from the US National Institutes of Health (NS43468 to M.D.), the National Institute of Mental Health (Silvio Conte Center for Neuroscience Research at UCSF to D.J.) and the Christopher Reeve Paralysis Foundation (to D.J.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Microglia from P2Y12-deficient mice have normal morphology and prevalence within the CNS. (PDF 15 kb)
Supplementary Fig. 2
Pretreatment with LPS diminishes ATP-induced microglial ruffling. (PDF 44 kb)
Supplementary Fig. 3
Microglia express P2Y13 transcripts, but not detectable receptor protein. (PDF 93 kb)
Supplementary Video 1
Wild-type (left) microglia in the Dunn chemotaxis chamber undergo membrane ruffling, polarization, and directed motility in the presence of a 50 to 0 μM ATP gradient (top to bottom) whereas P2Y12-deficient (right) microglia show dramatically reduced stimulation. The chemotaxis chamber was placed in a heated, C02 buffered humidified microscope incubator and phase contrast images were acquired every 150 s for 30 min. Scale bar represents 20 μm. (MOV 540 kb)
Supplementary Video 2
Acutely prepared hippocampal slices from P7 neonatal wild-type (left) and P2Y12-deficient (right) mice were bathed in imaging media with 1 mM ADP. Wild-type microglia respond within minutes by sending out long, cellular projections towards the periphery of the slice (top, right, and bottom edges of field of view). Some cells actually undergo whole-cell body displacement as they travel towards the nucleotide source while other cells extend elaborately branched ramifications. Some cells first respond by sending out branches before exhibiting whole cell locomotion. In striking contrast, microglia from P2Y12-deficient animals show a dramatically diminished behavioral response towards the nucleotide source. Images show the CA3 layer of the hippocampal slice. Cells were visualized by GFP expression, images were taken at 5 min intervals for 6.75 h, and a maximum projection of 15 z-steps spaced 2 μm apart (30 μm total thickness) was constructed for each frame. Scale bar represents 100 μm. (MOV 9980 kb)
Supplementary Video 3
In vivo imaging of GFP labelled microglia in wild-type (left) and P2Y12-deficient (right) mice after injection of 20 mM ATP (red needle). Wild-type microglia displayed robust branch extension towards the site of injection whereas P2Y12-deficient microglia did not. Experiment is 40 min, 4 min intervals. Scale bar represents 20 μm. (MOV 2432 kb)
Supplementary Video 4
In vivo imaging of GFP labelled microglia in wild-type (left) and P2Y12-deficient (right) mice after focal laser ablation induced by the two-photon laser. Wild-type microglia displayed robust process extension towards the site of injury whereas P2Y12-deficient microglia showed a dramatically reduced and time-delayed response. Experiment is 40 min, 4 min intervals. Scale bar represents 20 μm. (MOV 5589 kb)
Rights and permissions
About this article
Cite this article
Haynes, S., Hollopeter, G., Yang, G. et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 9, 1512–1519 (2006). https://doi.org/10.1038/nn1805
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1805
This article is cited by
-
Taming microglia: the promise of engineered microglia in treating neurological diseases
Journal of Neuroinflammation (2024)
-
A P2RY12 deficiency results in sex-specific cellular perturbations and sexually dimorphic behavioral anomalies
Journal of Neuroinflammation (2024)
-
Microglia regulate sleep through calcium-dependent modulation of norepinephrine transmission
Nature Neuroscience (2024)
-
The aging mouse CNS is protected by an autophagy-dependent microglia population promoted by IL-34
Nature Communications (2024)
-
Regulation of genes involved in the metabolic adaptation of murine microglial cells in response to elevated HIF-1α mediated activation
Immunogenetics (2024)