Abstract
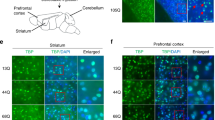
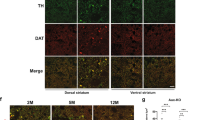
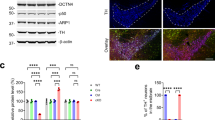
Non-neuronal cells may be pivotal in neurodegenerative disease, but the mechanistic basis of this effect remains ill-defined. In the polyglutamine disease spinocerebellar ataxia type 7 (SCA7), Purkinje cells undergo non-cell-autonomous degeneration in transgenic mice. We considered the possibility that glial dysfunction leads to Purkinje cell degeneration, and generated mice that express ataxin-7 in Bergmann glia of the cerebellum with the Gfa2 promoter. Bergmann glia–specific expression of mutant ataxin-7 was sufficient to produce ataxia and neurodegeneration. Expression of the Bergmann glia–specific glutamate transporter GLAST was reduced in Gfa2-SCA7 mice and was associated with impaired glutamate transport in cultured Bergmann glia, cerebellar slices and cerebellar synaptosomes. Ultrastructural analysis of Purkinje cells revealed findings of dark cell degeneration consistent with excitotoxic injury. Our studies indicate that impairment of glutamate transport secondary to glial dysfunction contributes to SCA7 neurodegeneration, and suggest a similar role for glial dysfunction in other polyglutamine diseases and SCAs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Clement, A.M. et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science 302, 113–117 (2003).
McGeer, P.L. et al. Microglia in degenerative neurological disease. Glia 7, 84–92 (1993).
Raeber, A.J. et al. Astrocyte-specific expression of hamster prion protein (PrP) renders PrP knockout mice susceptible to hamster scrapie. EMBO J. 16, 6057–6065 (1997).
Yazawa, I. et al. Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron 45, 847–859 (2005).
Forman, M.S. et al. Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration. J. Neurosci. 25, 3539–3550 (2005).
Voogd, J. & Glickstein, M. The anatomy of the cerebellum. Trends Neurosci. 21, 370–375 (1998).
Taroni, F. & DiDonato, S. Pathways to motor incoordination: the inherited ataxias. Nat. Rev. Neurosci. 5, 641–655 (2004).
Konigsmark, B.W. & Weiner, L.P. The olivopontocerebellar atrophies: a review. Medicine (Baltimore) 49, 227–241 (1970).
Zoghbi, H.Y. & Orr, H.T. Glutamine repeats and neurodegeneration. Annu. Rev. Neurosci. 23, 217–247 (2000).
Garden, G.A. et al. Polyglutamine-expanded ataxin-7 promotes non-cell-autonomous purkinje cell degeneration and displays proteolytic cleavage in ataxic transgenic mice. J. Neurosci. 22, 4897–4905 (2002).
La Spada, A.R. et al. Polyglutamine-expanded ataxin-7 antagonizes CRX function and induces cone-rod dystrophy in a mouse model of SCA7. Neuron 31, 913–927 (2001).
Borchelt, D.R. et al. A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet. Anal. 13, 159–163 (1996).
Gu, X. et al. Pathological cell-cell interactions elicited by a neuropathogenic form of mutant huntingtin contribute to cortical pathogenesis in HD mice. Neuron 46, 433–444 (2005).
Grosche, J. et al. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat. Neurosci. 2, 139–143 (1999).
Yamada, K. et al. Dynamic transformation of Bergmann glial fibers proceeds in correlation with dendritic outgrowth and synapse formation of cerebellar Purkinje cells. J. Comp. Neurol. 418, 106–120 (2000).
Brenner, M., Kisseberth, W.C., Su, Y., Besnard, F. & Messing, A. GFAP promoter directs astrocyte-specific expression in transgenic mice. J. Neurosci. 14, 1030–1037 (1994).
Brenner, M. & Messing, A. GFAP transgenic mice. Methods 10, 351–364 (1996).
Strahlendorf, J.C., Brandon, T., Miles, R. & Strahlendorf, H.K. AMPA receptor-mediated alterations of intracellular calcium homeostasis in rat cerebellar Purkinje cells in vitro: correlates to dark cell degeneration. Neurochem. Res. 23, 1355–1362 (1998).
Strahlendorf, J.C. & Strahlendorf, H.K. Enduring changes in Purkinje cell electrophysiology following transient exposure to AMPA: correlates to dark cell degeneration. Neurosci. Res. 33, 155–162 (1999).
Leranth, C. & Hamori, J. “Dark” Purkinje cells of the cerebellar cortex. Acta Biol. Acad. Sci. Hung. 21, 405–419 (1970).
Furuta, A., Rothstein, J.D. & Martin, L.J. Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J. Neurosci. 17, 8363–8375 (1997).
Yoo, S.Y. et al. SCA7 knockin mice model human SCA7 and reveal gradual accumulation of mutant ataxin-7 in neurons and abnormalities in short-term plasticity. Neuron 37, 383–401 (2003).
Cui, W., Allen, N.D., Skynner, M., Gusterson, B. & Clark, A.J. Inducible ablation of astrocytes shows that these cells are required for neuronal survival in the adult brain. Glia 34, 272–282 (2001).
Fields, R.D. & Stevens-Graham, B. New insights into neuron-glia communication. Science 298, 556–562 (2002).
Watase, K. et al. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. Eur. J. Neurosci. 10, 976–988 (1998).
Huang, H. & Bordey, A. Glial glutamate transporters limit spillover activation of presynaptic NMDA receptors and influence synaptic inhibition of Purkinje neurons. J. Neurosci. 24, 5659–5669 (2004).
Arriza, J.L. et al. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J. Neurosci. 14, 5559–5569 (1994).
Shin, J.Y. et al. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J. Cell Biol. 171, 1001–1012 (2005).
Iino, M. et al. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science 292, 926–929 (2001).
Gong, Y.H., Parsadanian, A.S., Andreeva, A., Snider, W.D. & Elliott, J.L. Restricted expression of G86R Cu/Zn superoxide dismutase in astrocytes results in astrocytosis but does not cause motoneuron degeneration. J. Neurosci. 20, 660–665 (2000).
Yvert, G. et al. Expanded polyglutamines induce neurodegeneration and trans-neuronal alterations in cerebellum and retina of SCA7 transgenic mice. Hum. Mol. Genet. 9, 2491–2506 (2000).
Probst-Cousin, S. et al. Spinocerebellar ataxia type 2 with glial cell cytoplasmic inclusions. J. Neurol. Neurosurg. Psychiatry 75, 503–505 (2004).
Palhan, V.B. et al. Polyglutamine-expanded ataxin-7 inhibits STAGA histone acetyltransferase activity to produce retinal degeneration. Proc. Natl. Acad. Sci. USA 102, 8472–8477 (2005).
Helmlinger, D. et al. Ataxin-7 is a subunit of GCN5 histone acetyltransferase-containing complexes. Hum. Mol. Genet. 13, 1257–1265 (2004).
La Spada, A.R. & Taylor, J.P. Polyglutamines placed into context. Neuron 38, 681–684 (2003).
Robinson, M.B. & Dowd, L.A. Heterogeneity and functional properties of subtypes of sodium-dependent glutamate transporters in the mammalian central nervous system. Adv. Pharmacol. 37, 69–115 (1997).
Gegelashvili, G. et al. Glutamate receptor agonists up-regulate glutamate transporter GLAST in astrocytes. Neuroreport 8, 261–265 (1996).
Duan, S., Anderson, C.M., Stein, B.A. & Swanson, R.A. Glutamate induces rapid upregulation of astrocyte glutamate transport and cell-surface expression of GLAST. J. Neurosci. 19, 10193–10200 (1999).
Lehre, K.P. & Danbolt, N.C. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J. Neurosci. 18, 8751–8757 (1998).
Dehnes, Y. et al. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J. Neurosci. 18, 3606–3619 (1998).
Lin, X., Antalffy, B., Kang, D., Orr, H.T. & Zoghbi, H.Y. Polyglutamine expansion down-regulates specific neuronal genes before pathologic changes in SCA1. Nat. Neurosci. 3, 157–163 (2000).
Serra, H.G. et al. Gene profiling links SCA1 pathophysiology to glutamate signaling in Purkinje cells of transgenic mice. Hum. Mol. Genet. 13, 2535–2543 (2004).
Ikeda, Y. et al. Spectrin mutations cause spinocerebellar ataxia type 5. Nat. Genet. 38, 184–190 (2006).
Rothstein, J.D., Van Kammen, M., Levey, A.I., Martin, L.J. & Kuncl, R.W. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann. Neurol. 38, 73–84 (1995).
Rothstein, J.D. et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 433, 73–77 (2005).
Lin, C.H. et al. Neurological abnormalities in a knock-in mouse model of Huntington's disease. Hum. Mol. Genet. 10, 137–144 (2001).
Ortega, A., Eshhar, N. & Teichberg, V.I. Properties of kainate receptor/channels on cultured Bergmann glia. Neuroscience 41, 335–349 (1991).
Shimada, F. et al. The neuroprotective agent MS-153 stimulates glutamate uptake. Eur. J. Pharmacol. 386, 263–270 (1999).
Lievens, J.C. et al. Impaired glutamate uptake in the R6 Huntington's disease transgenic mice. Neurobiol. Dis. 8, 807–821 (2001).
Acknowledgements
We would like to thank A.H. Koeppen (VA Medical Center, Albany, New York) with support from the National Ataxia Foundation for brain sections, E. Brunt for clinical assessment of patients, J. Huang, D.E. Possin, H. Korff, B. Meseck-Selchow, S. Baccam, V. Damian, C. Plata, A.C. Smith and B. Wang for technical assistance, and H. Zoghbi and J. Gatchel-Rose for providing the SCA7-266Q knock-in mice. This work was supported by funding from the US National Institutes of Health (EY14061 to A.R.L., P30 HD02774 from the National Institute of Child Health and Human Development, a National Organization for Rare Disease (NORD) grant to G.A.G. and a Neurobiology Predoctoral training grant to S.K.C.), the Deutsche Forschungsgemeinschaft (RU 1215/1-1), the Deutsche Heredo-Ataxie-Gesellschaft (DHAG), the ADCA-Vereniging Nederland and the Bernd Fink-Stiftung (Düsseldorf, Germany). A.R.L. is a recipient of a Paul Beeson Physician Faculty Scholar in Aging Research award from the American Foundation for Aging Research (AFAR).
Author information
Authors and Affiliations
Contributions
S.K.C. and B.L.S. conducted the Gfa2 transgenic studies and glutamate transporter studies. G.A.G. performed the histology analysis. G.A.G., N.G. and L.E.W. performed the ultrastructural analysis. R.T.L. and S.J.G. carried out the expression analysis. U.R., C.S. and T.D. conducted the histology analysis. A.R.L.S. directed all studies.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Bergmann glia cell bodies ensheath Purkinje cells. (PDF 138 kb)
Supplementary Fig. 2
Validation of Gfa2-SCA7 expression constructs in Bergmann glia and in transgenic mice. (PDF 7540 kb)
Supplementary Fig. 3
Morphological criteria for ultrastructural assessment of Purkinje cell dendrites. (PDF 181 kb)
Supplementary Fig. 4
PrP-SCA7-92Q mice display Bergmann glia pathology. (PDF 622 kb)
Supplementary Fig. 5
Dramatic degeneration of Bergmann glia in the high-expressing Gfa2-SCA7-92Q line. (PDF 4705 kb)
Supplementary Fig. 6
Western blot analysis of GLAST and GLT-1 protein expression in Gfa2-SCA7 transgenic mice at various ages. (PDF 627 kb)
Supplementary Fig. 7
PrP-SCA7-92Q Bergmann glia display reduced GLAST levels. (PDF 43 kb)
Rights and permissions
About this article
Cite this article
Custer, S., Garden, G., Gill, N. et al. Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat Neurosci 9, 1302–1311 (2006). https://doi.org/10.1038/nn1750
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1750
This article is cited by
-
Reactive Bergmann glia play a central role in spinocerebellar ataxia inflammation via the JNK pathway
Journal of Neuroinflammation (2023)
-
Contribution of Glial Cells to Polyglutamine Diseases: Observations from Patients and Mouse Models
Neurotherapeutics (2023)
-
Polyglutamine-expanded ATXN7 alters a specific epigenetic signature underlying photoreceptor identity gene expression in SCA7 mouse retinopathy
Journal of Biomedical Science (2022)
-
Consensus Paper: Strengths and Weaknesses of Animal Models of Spinocerebellar Ataxias and Their Clinical Implications
The Cerebellum (2022)
-
ATG4D is the main ATG8 delipidating enzyme in mammalian cells and protects against cerebellar neurodegeneration
Cell Death & Differentiation (2021)