Abstract
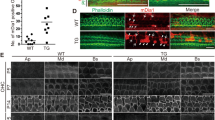
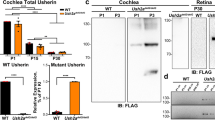
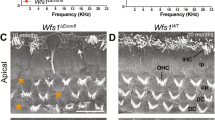
α-tectorin (encoded by Tecta) is a component of the tectorial membrane, an extracellular matrix of the cochlea. In humans, the Y1870C missense mutation in TECTA causes a 50- to 80-dB hearing loss. In transgenic mice with the Y1870C mutation in Tecta, the tectorial membrane's matrix structure is disrupted, and its adhesion zone is reduced in thickness. These abnormalities do not seriously influence the tectorial membrane's known role in ensuring that cochlear feedback is optimal, because the sensitivity and frequency tuning of the mechanical responses of the cochlea are little changed. However, neural thresholds are elevated, neural tuning is broadened, and a sharp decrease in sensitivity is seen at the tip of the neural tuning curve. Thus, using TectaY1870C/+ mice, we have genetically isolated a second major role for the tectorial membrane in hearing: it enables the motion of the basilar membrane to optimally drive the inner hair cells at their best frequency.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lim, D.J. Functional structure of the organ of Corti: a review. Hear. Res. 22, 117–146 (1986).
Brownell, W.E., Bader, C.R., Betrand, D. & de Ribaupierre, Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science 227, 194–196 (1985).
Patuzzi, R.B., Yates, G.K. & Johnstone, B.M. Outer hair receptor currents and sensorineural hearing loss. Hear. Res. 42, 47–72 (1989).
Ruggero, M.A. & Rich, N.C. Furosemide alters organ of Corti mechanics: evidence for feedback of outer hair cells upon the basilar membrane. J. Neurosci. 11, 1057–1067 (1991).
Kössl, M. & Russell, I.J. The phase and magnitude of hair cell receptor potentials and frequency tuning in the guinea pig cochlea. J. Neurosci. 12, 1575–1586 (1992).
Spoendlin, H. Anatomy of cochlear innervation. Am. J. Otolaryngol. 6, 453–467 (1985).
Kimura, R.S. Hairs of the cochlear sensory cells and their attachment to the tectorial membrane. Acta Otolaryngol. (Stockh.) 61, 55–72 (1966).
Hasko, J.A. & Richardson, G.P. The ultrastructural organization and properties of the mouse tectorial membrane matrix. Hear. Res. 35, 21–38 (1988).
Richardson, G.P., Russell, I.J., Duance, V.C. & Bailey, A.J. Polypeptide composition of the mammalian tectorial membrane. Hear. Res. 25, 45–60 (1987).
Thalmann, I. et al. Composition and supramolecular organization of the tectorial membrane. Laryngoscope 97, 357–367 (1987).
Legan, P.K., Rau, A., Keen, J.N. & Richardson, G.P. The mouse tectorins: modular matrix proteins of the inner ear homologous to components of the sperm-egg adhesion system. J. Biol. Chem. 272, 8791–8801 (1997).
Cohen-Salmon, M., El-Amraoui, A., Leibovici, M. & Petit, C. Otogelin: a glycoprotein specific to the acellular membranes of the inner ear. Proc. Natl. Acad. Sci. USA 94, 14450–14455 (1997).
Verhoeven, K. et al. Mutations in human α-tectorin cause autosomal dominant non-syndromic hearing impairment. Nat. Genet. 19, 60–62 (1998).
Davis, H. Transmission and transduction in the cochlea. Laryngoscope 68, 359–382 (1957).
Zwislocki, J.J. Theory of cochlear mechanics. Hear. Res. 2, 171–182 (1980).
Zwislocki, J.J. Analysis of cochlear mechanics. Hear. Res. 22, 155–169 (1986).
Allen, J.B. Cochlear micromechanics - a physical model of transduction. J. Acoust. Soc. Am. 68, 1660–1670 (1980).
Legan, P.K. et al. A targeted deletion in α-tectorin reveals the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron 28, 273–285 (2000).
Gummer, A.W., Hemmert, W. & Zenner, H.P. Resonant tectorial membrane motion in the inner ear: Its crucial role in frequency tuning. Proc. Natl. Acad. Sci. USA 93, 8727–8732 (1996).
Hemmert, W., Zenner, H.P. & Gummer, A.W. Three-dimensional motion of the organ of Corti. Biophys. J. 78, 2285–2297 (2000).
Lukashkin, A.N., Lukashkina, V.A., Legan, P.K., Richardson, G.P. & Russell, I.J. Role of the tectorial membrane revealed by otoacoustic emissions recorded from wild-type and transgenic Tecta (deltaENT/deltaENT) mice. J. Neurophysiol. 91, 163–171 (2004).
Lin, T. & Guinan, J.J. Auditory-nerve-fiber responses to high-level clicks: Interference patterns indicate that excitation is due to the combination of multiple drives. J. Acoust. Soc. Am. 107, 2615–2630 (2000).
Dallos, P., Billone, M.C., Durrant, J.D., Wang, C. & Raynor, S. Cochlear inner and outer hair cells: functional differences. Science 177, 356–358 (1972).
Russell, I.J. & Sellick, P.M. Low-frequency characteristics of intracellularly recorded receptor potentials in guinea-pig cochlear hair cells. J. Physiol. (Lond.) 338, 179–206 (1983).
Patuzzi, R.B. & Yates, G.K. The low-frequency response of inner hair cells in the guinea pig cochlea: implications for fluid coupling and resonance of the stereocilia. Hear. Res. 30, 83–98 (1987).
Aranyosi, A.J. & Freemann, D.M. Sound-induced motions of individual cochlear hair bundles. Biophys. J. 87, 3536–3546 (2004).
Russell, I.J., Kössl, M. & Murugasu, E. A comparison between tone-evoked voltage responses of hair cells and basilar membrane displacements recorded in the basal turn of the guinea pig cochlea. in Advances in Hearing Research (eds. Manley, G.A., Klump, G.M., Köppl, C., Fastl, H. & Oeckinghaus, H.) 125–135 (World Scientific, Singapore, 1995).
Narayan, S.S., Temchin, A.N., Recio, A. & Ruggero, M.A. Frequency tuning of basilar membrane and auditory nerve fibers in the same cochleae. Science 282, 1882–1884 (1998).
Kletsky, E.J. & Zwislocki, J.J. CM tuning can be compatible with sharply tuned receptor potentials. Hear. Res. 2, 549–557 (1980).
Frolenkov, G.I., Belyantseva, I.A., Kurc, M., Mastroianni, M.A. & Kachar, B. Cochlear outer hair cell electromotility can provide force for both low and high intensity distortion product otoacoustic emissions. Hear. Res. 126, 67–74 (1998).
Lukashkin, A.N., Lukashkina, V.A. & Russell, I.J. One source for distortion product otoacoustic emissions generated by low- and high-level primaries. J. Acoust. Soc. Am. 111, 2740–2748 (2002).
Santos-Sacchi, J. Harmonics of outer hair cell motility. Biophys. J. 65, 2217–2227 (1993).
Lukashkin, A.N. & Russell, I.J. A descriptive model of the receptor potential nonlinearities generated by the hair cell mechanoelectrical transducer. J. Acoust. Soc. Am. 103, 973–980 (1998).
Mom, T., Telischi, F.F., Martin, G.K. & Lonsbury-Martin, B.L. Measuring the cochlear blood flow and distortion-product otoacoustic emissions during reversible cochlear ischemia: a rabbit model. Hear. Res. 133, 40–52 (1999).
Robertson, D. Horseradish peroxidase injection of physiologically characterized afferent and efferent neurones in the guinea pig spiral ganglion. Hear. Res. 15, 113–121 (1984).
Davis, H., Tasaki, I. & Goldstein, R. The peripheral origin of activity, with reference to the ear. Cold Spring Harb. Symp. Quant. Biol. 17, 143–154 (1952).
Sellick, P., Patuzzi, R. & Robertson, D. Primary afferent and cochlear nucleus contributions to extracellular potentials during tone-bursts. Hear. Res. 176, 42–58 (2003).
Dallos, P. & Cheatham, M.A. Compound action potential (AP) tuning curves. J. Acoust. Soc. Am. 59, 591–597 (1976).
Jovine, L., Qi, H., Williams, Z. & Wassarman, P.M. The ZP domain is a conserved module for polymerisation of extracellular proteins. Nat. Cell Biol. 4, 457–461 (2002).
Bork, P. & Sander, C. A large domain common to sperm receptors (Zp2 and Zp3) and TGF-beta type III receptor. FEBS Lett. 300, 237–240 (1992).
Wassarman, P.M., Jovine, L. & Litscher, E.S. A profile of fertilization in mammals. Nat. Cell Biol. 3, E59–E64 (2001).
Mustapha, M. et al. An alpha-tectorin gene defect causes a newly identified autosomal recessive form of sensorineural pre-lingual non-syndromic deafness, DFNB21. Hum. Mol. Genet. 8, 409–412 (1999).
Liberman, M.C. & Kiang, N.Y.-S. Acoustic trauma in cats. Acta Otolaryngol. Suppl. 358, 1–63 (1978).
Robles, L. & Ruggero, M.A. Mechanics of the mammalian cochlea. Physiol. Rev. 81, 1305–1352 (2001).
Brookes, A.J., Stevenson, B.J., Porteous, D.J. & Dorin, J.R. A series of vectors that simplify mammalian gene targeting. Transgenic Res. 2, 238–244 (1993).
Malumbres, M., Mangues, R., Ferrer, N., Lu, S. & Pellicer, C. Isolation of high molecular weight DNA for reliable genotyping of transgenic mice. Biotechniques 22, 1114–1119 (1997).
Knipper, M. et al. Thyroid hormone-deficient period prior to the onset of hearing is associated with reduced levels of beta-tectorin protein in the tectorial membrane: implication for hearing loss. J. Biol. Chem. 276, 39046–39052 (2001).
Russell, I.J. & Kössl, M. Micromechanical responses to tones in the auditory fovea of the greater mustached bat's cochlea. J. Neurophysiol. 82, 676–686 (1999).
Acknowledgements
The authors would like to thank M. Mellado, M. Kössl and M. Drexl for their helpful criticisms of the manuscript and J. Hartley for expert technical assistance. Supported by grants from The Wellcome Trust, Defeating Deafness and the Fonds Wetenschappelijk Onderzoek – Flanders.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Legan, P., Lukashkina, V., Goodyear, R. et al. A deafness mutation isolates a second role for the tectorial membrane in hearing. Nat Neurosci 8, 1035–1042 (2005). https://doi.org/10.1038/nn1496
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1496
This article is cited by
-
Distortion Product Otoacoustic Emissions in Mice Above and Below the Eliciting Primaries
Journal of the Association for Research in Otolaryngology (2023)
-
Reducing tectorial membrane viscoelasticity enhances spontaneous otoacoustic emissions and compromises the detection of low level sound
Scientific Reports (2019)
-
Prevalence of TECTA mutation in patients with mid-frequency sensorineural hearing loss
Orphanet Journal of Rare Diseases (2017)
-
A connexin30 mutation rescues hearing and reveals roles for gap junctions in cochlear amplification and micromechanics
Nature Communications (2017)
-
Genetics of auditory mechano-electrical transduction
Pflügers Archiv - European Journal of Physiology (2015)