Abstract
The cerebellum is critical for motor coordination and cognitive function and is the target of transformation in medulloblastoma, the most common malignant brain tumor in children. Although the development of granule cells, the most abundant neurons in the cerebellum, has been studied in detail, the origins of other cerebellar neurons and glia remain poorly understood. Here we show that the murine postnatal cerebellum contains multipotent neural stem cells (NSCs). These cells can be prospectively isolated based on their expression of the NSC marker prominin-1 (CD133) and their lack of markers of neuronal and glial lineages (lin−). Purified prominin+lin− cells form self-renewing neurospheres and can differentiate into astrocytes, oligodendrocytes and neurons in vitro. Moreover, they can generate each of these lineages after transplantation into the cerebellum. Identification of cerebellar stem cells has important implications for the understanding of cerebellar development and the origins of medulloblastoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Rapoport, M., van Reekum, R. & Mayberg, H. The role of the cerebellum in cognition and behavior: a selective review. J. Neuropsychiatry Clin. Neurosci. 12, 193–198 (2000).
Altman, J. & Bayer, S.A. Development of the Cerebellar System: In Relation to Its Evolution, Structure and Functions (CRC Press, Boca Raton, Florida, USA, 1997).
Rosenberg, R.N. & Grossman, A. Hereditary ataxia. Neurol. Clin. 7, 25–36 (1989).
Kern, J.K. The possible role of the cerebellum in autism/PDD: disruption of a multisensory feedback loop. Med. Hypotheses 59, 255–260 (2002).
Martin, P. & Albers, M. Cerebellum and schizophrenia: a selective review. Schizophr. Bull. 21, 241–250 (1995).
Wechsler-Reya, R. & Scott, M.P. The developmental biology of brain tumors. Annu. Rev. Neurosci. 24, 385–428 (2001).
Wetmore, C. Sonic hedgehog in normal and neoplastic proliferation: insight gained from human tumors and animal models. Curr. Opin. Genet. Dev. 13, 34–42 (2003).
Zhang, L. & Goldman, J.E. Generation of cerebellar interneurons from dividing progenitors in white matter. Neuron 16, 47–54 (1996).
Hallonet, M.E., Teillet, M.A. & Le Douarin, N.M. A new approach to the development of the cerebellum provided by the quail-chick marker system. Development 108, 19–31 (1990).
Lumpkin, E.A. et al. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr. Patterns 3, 389–395 (2003).
Wechsler-Reya, R.J. & Scott, M.P. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron 22, 103–114 (1999).
Cheng, Y., Tao, Y., Black, I.B. & DiCicco-Bloom, E. A single peripheral injection of basic fibroblast growth factor (bFGF) stimulates granule cell production and increases cerebellar growth in newborn rats. J. Neurobiol. 46, 220–229 (2001).
Wernecke, H., Lindner, J. & Schachner, M. Cell type specificity and developmental expression of the L2/HNK-1 epitopes in mouse cerebellum. J. Neuroimmunol. 9, 115–130 (1985).
Theodosis, D.T., Rougon, G. & Poulain, D.A. Retention of embryonic features by an adult neuronal system capable of plasticity: polysialylated neural cell adhesion molecule in the hypothalamo-neurohypophysial system. Proc. Natl. Acad. Sci. USA 88, 5494–5498 (1991).
Tucker, R.P., Binder, L.I., Viereck, C., Hemmings, B.A. & Matus, A.I. The sequential appearance of low- and high-molecular-weight forms of MAP2 in the developing cerebellum. J. Neurosci. 8, 4503–4512 (1988).
Sommer, I. & Schachner, M. Monoclonal antibodies (O1 to O4) to oligodendrocyte surfaces: an immunocytological study in the central nervous system. Dev. Biol. 83, 311–327 (1981).
Dawson, M.R., Polito, A., Levine, J.M. & Reynolds, R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci. 24, 476–488 (2003).
Bignami, A., Eng, L.F., Dahl, D. & Uyeda, C.T. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 43, 429–435 (1972).
Geisert, E.E., Jr., Yang, L. & Irwin, M.H. Astrocyte growth, reactivity, and the target of the antiproliferative antibody, TAPA. J. Neurosci. 16, 5478–5487 (1996).
Haegel, H., Tolg, C., Hofmann, M. & Ceredig, R. Activated mouse astrocytes and T cells express similar CD44 variants. Role of CD44 in astrocyte/T cell binding. J. Cell Biol. 122, 1067–1077 (1993).
Lendahl, U., Zimmerman, L.B. & McKay, R.D. CNS stem cells express a new class of intermediate filament protein. Cell 60, 585–595 (1990).
Corbeil, D., Roper, K., Fargeas, C.A., Joester, A. & Huttner, W.B. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic 2, 82–91 (2001).
Graham, V., Khudyakov, J., Ellis, P. & Pevny, L. SOX2 functions to maintain neural progenitor identity. Neuron 39, 749–765 (2003).
Sakakibara, S. et al. Mouse-Musashi-1, a neural RNA-binding protein highly enriched in the mammalian CNS stem cell. Dev. Biol. 176, 230–242 (1996).
Sawamoto, K. et al. Generation of dopaminergic neurons in the adult brain from mesencephalic precursor cells labeled with a nestin-GFP transgene. J. Neurosci. 21, 3895–3903 (2001).
Tamaki, S. et al. Engraftment of sorted/expanded human central nervous system stem cells from fetal brain. J. Neurosci. Res. 69, 976–986 (2002).
Reynolds, B.A. & Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255, 1707–1710 (1992).
Imura, T., Kornblum, H.I. & Sofroniew, M.V. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J. Neurosci. 23, 2824–2832 (2003).
Takahashi, J., Palmer, T.D. & Gage, F.H. Retinoic acid and neurotrophins collaborate to regulate neurogenesis in adult-derived neural stem cell cultures. J. Neurobiol. 38, 65–81 (1999).
Galli, R., Pagano, S.F., Gritti, A. & Vescovi, A.L. Regulation of neuronal differentiation in human CNS stem cell progeny by leukemia inhibitory factor. Dev. Neurosci. 22, 86–95 (2000).
Erlandsson, A., Enarsson, M. & Forsberg-Nilsson, K. Immature neurons from CNS stem cells proliferate in response to platelet-derived growth factor. J. Neurosci. 21, 3483–3491 (2001).
Aoki, E., Semba, R. & Kashiwamata, S. New candidates for GABAergic neurons in the rat cerebellum: an immunocytochemical study with anti-GABA antibody. Neurosci. Lett. 68, 267–271 (1986).
Simmons, M.L. & Dutton, G.R. Neuronal origins of K+-evoked amino acid release from cerebellar cultures. J. Neurosci. Res. 31, 646–653 (1992).
Maricich, S.M. & Herrup, K. Pax-2 expression defines a subset of GABAergic interneurons and their precursors in the developing murine cerebellum. J. Neurobiol. 41, 281–294 (1999).
Aruga, J. et al. A novel zinc finger protein, zic, is involved in neurogenesis, especially in the cell lineage of cerebellar granule cells. J. Neurochem. 63, 1880–1890 (1994).
Shetty, A.K. & Turner, D.A. In vitro survival and differentiation of neurons derived from epidermal growth factor-responsive postnatal hippocampal stem cells: inducing effects of brain-derived neurotrophic factor. J. Neurobiol. 35, 395–425 (1998).
Laywell, E.D., Rakic, P., Kukekov, V.G., Holland, E.C. & Steindler, D.A. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc. Natl. Acad. Sci. USA 97, 13883–13888 (2000).
Singh, S.K. et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 63, 5821–5828 (2003).
Gabay, L., Lowell, S., Rubin, L.L. & Anderson, D.J. Deregulation of dorsoventral patterning by FGF confers trilineage differentiation capacity on CNS stem cells in vitro. Neuron 40, 485–499 (2003).
Gao, W.Q. & Hatten, M.E. Immortalizing oncogenes subvert the establishment of granule cell identity in developing cerebellum. Development 120, 1059–1070 (1994).
Snyder, E.Y. et al. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell 68, 33–51 (1992).
Milosevic, A. & Goldman, J.E. Progenitors in the postnatal cerebellar white matter are antigenically heterogeneous. J. Comp. Neurol. 452, 192–203 (2002).
Alder, J., Cho, N.K. & Hatten, M.E. Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron 17, 389–399 (1996).
Zinyk, D.L., Mercer, E.H., Harris, E., Anderson, D.J. & Joyner, A.L. Fate mapping of the mouse midbrain-hindbrain constriction using a site-specific recombination system. Curr. Biol. 8, 665–668 (1998).
Pietsch, T. et al. Medulloblastomas of the desmoplastic variant carry mutations of the human homologue of Drosophila patched. Cancer Res. 57, 2085–2088 (1997).
Pomeroy, S.L. et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature 415, 436–442 (2002).
Katsetos, C.D. et al. Calbindin-D28k in subsets of medulloblastomas and in the human medulloblastoma cell line D283 Med. Arch. Pathol. Lab. Med. 119, 734–743 (1995).
Hemmati, H.D. et al. Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl. Acad. Sci. USA 100, 15178–15183 (2003).
Weigmann, A., Corbeil, D., Hellwig, A. & Huttner, W.B. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc. Natl. Acad. Sci. USA 94, 12425–12430 (1997).
Groszer, M. et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science 294, 2186–2189 (2001).
Acknowledgements
The authors thank M. Cook for flow cytometric analysis; E. Snyder and J.-P. Lee for assistance with intracranial injections; W. Salmon for help with microscopy; and A. Shetty, B. Barres and M. Rao for helpful discussions. R.W.R. is a Kimmel Foundation Scholar. This research was supported by a McDonnell Foundation 21st Century Award and by grant #1R01MH067916-01 from the US National Institute of Mental Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
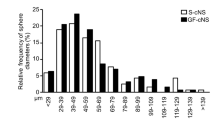
Purification of cerebellar stem cells and assessment of neurosphere forming-efficiency. To enrich for stem cells, cells from the cerebellum of Math1-GFP mice (cerebellar suspension) were stained with antibodies specific for Prominin and for neuronal and glial lineage markers (O4, TAPA-1 and PSA-NCAM). Cells were then FACS-sorted into GFP+ (GCP) and GFP– (non-GCP) fractions, representing 90% and 10% of the starting population respectively. GFP– cells were further fractionated into Prominin– cells (7% of the cerebellar suspension), Prominin+Lineage+ cells (2.8%) and Prominin+Lineage– cells (0.2%). Each of these fractions was cultured at clonal density in bFGF and EGF for 10 days, and then the neurosphere forming efficiency (neurospheres/cells plated, shown in orange boxes) was determined. The starting cerebellar suspension formed one neurosphere for every 1000 cells plated. GCPs (Math1-GFP+ cells) could not form neurospheres when cultured at clonal density in the presence of bFGF + EGF or Sonic hedgehog; in contrast, Math1-GFP– cells showed a 10-fold enrichment in neurosphere-forming ability (1 neurosphere/100 cells) compared to the starting cell suspension. Prominin– cells survived poorly and did not form neurospheres at clonal density. Prominin+Lineage+ cells formed few free-floating neurospheres (<1 neurosphere/100 cells), but often gave rise to adherent colonies that underwent neuronal differentiation. Prominin+Lineage– cells formed one neurosphere for every 30 cells plated. (PDF 502 kb)
Supplementary Fig. 2
Self-renewal of cerebellar stem cells. (a) Primary and secondary neurospheres. FACS-sorted Prominin+Lin– cells were cultured at clonal density in media containing bFGF and EGF. After 10 days, primary (1°) neurospheres were photographed at 20X magnification. Primary neurospheres were then dissociated and replated under the conditions described above. Secondary (2°) neurospheres, photographed at 10 days, resembled primary neurospheres in morphology and frequency. Neurospheres could be propagated in this manner for up to 10 weeks in vitro. (b) Efficiency of neurosphere formation at clonal density and after repeated passage in culture. The efficiency of neurosphere formation was similar whether Prominin+Lineage– cells were cultured at a density of 1 cell per well or at a density of 1 cell per mm2 (clonal density). Moreover, neurosphere-forming efficiency was maintained in successive passages of neurospheres generated at clonal density. In all cases, approximately 1 neurosphere was generated for every 30 cells plated. (PDF 681 kb)
Rights and permissions
About this article
Cite this article
Lee, A., Kessler, J., Read, TA. et al. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci 8, 723–729 (2005). https://doi.org/10.1038/nn1473
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1473
This article is cited by
-
Emerging roles of prominin-1 (CD133) in the dynamics of plasma membrane architecture and cell signaling pathways in health and disease
Cellular & Molecular Biology Letters (2024)
-
Marinopyrrole derivative MP1 as a novel anti-cancer agent in group 3 MYC-amplified Medulloblastoma
Journal of Experimental & Clinical Cancer Research (2024)
-
Loss of phosphatase CTDNEP1 potentiates aggressive medulloblastoma by triggering MYC amplification and genomic instability
Nature Communications (2023)
-
Cisplatin selects for CD133+ cells in lung cancer cells
Oncology and Translational Medicine (2020)
-
Allopregnanolone Promotes Neuronal and Oligodendrocyte Differentiation In Vitro and In Vivo: Therapeutic Implication for Alzheimer's Disease
Neurotherapeutics (2020)