Abstract
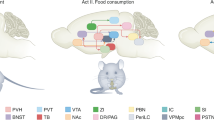
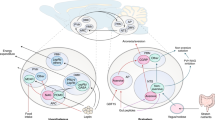
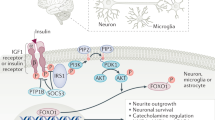
Food intake and energy expenditure are determinants of metabolic phenotype and are regulated by the CNS. Although humans have a well-balanced homeostatic feedback loop, obesity and metabolic disorders are spreading rapidly and carry a heavy toll of morbidity and mortality. The past decade has witnessed major advances in the understanding of basic metabolic processes, the brain circuitry that determines appropriate and, but, inappropriate behavioral and humoral responses to changing metabolic cues remains largely ill defined. This review summarizes current knowledge of the brain anatomy that supports food intake and energy expenditure and discusses cellular mechanisms such as synaptic plasticity that may provide clues toward the development of successful central therapies to combat metabolic disorders, including obesity and diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Friedman, J.M. & Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 395, 763–770 (1998).
Elmquist, J.K., Elias, C.F. & Saper, C.B. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 22, 221–232 (1999).
Kalra, S.P. et al. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr. Rev. 20, 68–100 (1999).
Schwartz, M.W., Woods, S.C., Porte, D. Jr, Seeley, R.J. & Baskin, D.G. Central nervous system control of food intake. Nature 404, 661–671 (2000).
Sherrington, C. Sir . Cutaneous sensation. in Textbook of Physiology (ed. Sharpey-Schaefer, E.A.) 920–1001 (Pentland, Edinburgh, 1900).
Cannon, W.B. & Washburn, A.L. An explanation of hunger. Am. J. Physiol. 29, 441 (1912).
Carlson, A.J. in Control of Hunger in Health and Disease 2nd impr. (Univ. of Chicago Press, Chicago, 1916).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Halaas, J.L. et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546 (1995).
Pelleymounter, M.A. et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269, 540–543 (1995).
Campfield, L.A., Smith, F.J., Guisez, Y., Devos, R. & Burn, P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269, 546–549 (1995).
Tartaglia, L.A. et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 83, 1263–1271 (1995).
Mercer, J.G. et al. Localization of leptin receptor mRNA and the long splice variant (O-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 387, 113–116 (1996).
Schwartz, M.W., Seeley, R.J., Campfield, L.A., Burn, P. & Baskin, D.G. Identification of leptin action in rat hypothalamus. J. Clin. Invest. 98, 1101–1106 (1996).
Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 (1997).
Fan, W., Boston, B.A., Kesterson, R.A., Hruby, V.J. & Cone, R.D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385, 165–168 (1997).
Pelletier, G. & Dube, D. Electron microscopic immunohistochemical localization of α-msh in the rat brain. Am. J. Anat. 150, 201–205 (1977).
Seeley, R.J. et al. Melanocortin receptors in leptin effects. Nature 390, 349 (1997).
Ollmann, M.M. et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278, 135–138 (1997).
Hahn, T.M., Breininger, J.F., Baskin, D.G. & Schwartz, M.W. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat. Neurosci. 1, 271–272 (1998).
Elias, C.F. et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 23, 775–786 (1999).
Cowley, M.A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001).
Horvath, T.L., Naftolin, F., Kalra, S.P. & Leranth, C. Neuropeptide-Y innervation of beta-endorphin-containing cells in the rat mediobasal hypothalamus: a light and electron microscopic double immunostaining analysis. Endocrinology 131, 2461–2467 (1992).
Pinto, S. et al. Rapid re-wiring of arcuate nucleus feeding circuits by leptin. Science 304, 110–115 (2004).
Cowley, M.A. Hypothalamic melanocortin neurons integrate signals of energy state. Eur. J. Pharmacol. 480, 3–11 (2003).
Broberger, C., Johansen, J., Johansson, C., Schalling, M. & Hokfelt, T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc. Natl. Acad. Sci. USA 95, 15043–15048 (1998).
Mezey, E. et al. Distribution of the pro-opiomelanocortin derived peptides, adrenocorticotrope hormone, α-melanocyte-stimulating hormone and β-endorphin (ACTH, α-MSH, β-END) in the rat hypothalamus. Brain Res. 328, 341–347 (1985).
Elias, C.F. et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21, 1375–1385 (1998).
Qu, D. et al. A role for melanin-concentrating hormone in the central regulation of feeding behavior. Nature 380, 243–247 (1996).
Sakurai, T. et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 (1998).
Theodosis, D.T. & Poulain, D.A. Evidence for structural plasticity in the supraoptic nucleus of the rat hypothalamus in relation to gestation and lactation. Neuroscience 11, 183–193 (1984).
Garcia-Segura, L.M., Baetens, D. & Naftolin, F. Synaptic remodelling in arcuate nucleus after injection of estradiol valerate in adult female rats. Brain Res. 366, 131–136 (1986).
Horvath, T.L. & Gao, X.B. Input organization and plasticity of hypocretin neurons: possible clues for obesity's association with insomnia. Cell Metabolism 1, 279–286 (2005).
Horvath, T.L. & Diano, S. The floating blueprint of hypothalamic feeding circuits. Nat. Rev. Neurosci. 5, 662–667 (2004).
Grill, H.J. & Kaplan, J.M. The neuroanatomical axis for control of energy balance. Front. Neuroendocrinol. 23, 2–40 (2002).
Bodosi, B. et al. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R1071–R1079 (2004).
Bouret, S.G., Draper, S.J. & Simerly, R.B. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304, 108–110 (2004).
Chemelli, R.M. et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98, 437–451 (1999).
Yamanaka, A. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 38, 701–713 (2003).
Li, Y., Gao, X.B., Sakurai, T. & van den Pol, A.N. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron 36, 1169–1181 (2002).
Shepherd, G.M. Introduction to synaptic circuits. in The Synaptic Organization of the Brain. (ed. Shepherd, G.M.) 1–38 (Oxford Univ. Press, New York, 2004).
Taheri, S., Lin, L., Austin, D., Young, T., Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med., published online 7 December 2004 (10.1371/journal.pmed.0010062).
Spiegel, K., Tasali, E., Penev, P. & Van Cauter, E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 141, 846–850 (2004).
Flier, J.S. & Elmquist, J.K. A good night's sleep: future antidote to the obesity epidemic? Ann. Intern. Med. 141, 885–886 (2004).
Pittenger, C. & Kandel, E.R. In search of general mechanisms for long-lasting plasticity: Aplysia and the hippocampus. Phil. Trans. R. Soc. Lond. B 358, 757–763 (2003).
Malenka, R.C. & Bear, M.F. LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 (2004).
Cooke, S.F. & Bliss, T.V. Long-term potentiation and cognitive drug discovery. Curr. Opin. Investig. Drugs 6, 25–34 (2005).
Schally, A.V. et al. Basic and clinical studies with luteinizing hormone-releasing hormone (LH-RH) and its analogues. J. Reprod. Fertil. Suppl. 20, 119–136 (1973).
Pfaff, D.W. et al. GnRH neurons and other cellular and molecular mechanisms for simple mammalian reproductive behaviors. Recent Prog. Horm. Res. 49, 1–25 (1994).
Acknowledgements
The work of T.L.H. on the relationship between synaptic plasticity and energy metabolism has been supported by National Institutes of Health grants DK-060711 and RR-014451.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Horvath, T. The hardship of obesity: a soft-wired hypothalamus. Nat Neurosci 8, 561–565 (2005). https://doi.org/10.1038/nn1453
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1453
This article is cited by
-
The hypothalamus for whole-body physiology: from metabolism to aging
Protein & Cell (2022)
-
Changes in body composition in unilateral vestibular hypofunction: relationships between bioelectrical impedance analysis and neuro-otological parameters
European Archives of Oto-Rhino-Laryngology (2021)
-
Functional interaction between Ghrelin and GLP-1 regulates feeding through the vagal afferent system
Scientific Reports (2020)
-
Association between childhood trauma and risk for obesity: a putative neurocognitive developmental pathway
BMC Medicine (2020)
-
The hypothalamus to brainstem circuit suppresses late-onset body weight gain
Scientific Reports (2019)