Abstract
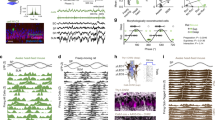
Oscillatory spike timing in the hippocampus is regarded as a temporal coding mechanism for space, but the underlying mechanisms are poorly understood. To contrast the predictions of the different models of phase precession, we transiently turned off neuronal discharges for up to 250 ms and reset the phase of theta oscillations by stimulating the commissural pathway in rats. After recovery from silence, phase precession continued. The phase of spikes for the first theta cycle after the perturbation was more advanced than the phase of spikes for the last theta cycle just before the perturbation. These findings indicate that phase advancement that emerges within hippocampal circuitry may be updated at the beginning of each theta cycle by extrahippocampal inputs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
O'Keefe, J. & Dostrovsky, J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175 (1971).
O'Keefe, J. & Recce, M.L. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3, 317–330 (1993).
Skaggs, W.E., McNaughton, B.L., Wilson, M.A. & Barnes, C.A. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6, 149–172 (1996).
Harris, K.D., Henze, D.A., Hirase, H., Leinekugel, X., Dragoi, G., Czurko, A. & Buzsáki, G. Spike train dynamics predicts theta-related phase precession in hippocampal pyramidal cells. Nature 417, 738–741 (2002).
Mehta, M.R., Lee, A.K. & Wilson, M.A. Role of experience and oscillations in transforming a rate code into a temporal code. Nature 417, 741–746 (2002).
Huxter, J., Burgess, N. & O'Keefe, J. Independent rate and temporal coding in hippocampal pyramidal cells. Nature 425, 828–832 (2003).
Dragoi, G., Harris, K.D. & Buzsáki, G. Place representation within hippocampal networks is modified by long-term potentiation. Neuron 39, 843–853 (2003).
Jensen, O. & Lisman, J.E. Position reconstruction from an ensemble of hippocampal place cells: contribution of theta phase coding. J. Neurophysiol. 83, 2602–2609 (2000).
Jensen, O. & Lisman, J.E. Hippocampal CA3 region predicts memory sequences: accounting for the phase precession of place cells. Learn. Mem. 3, 279–287 (1996).
Lisman, J.E. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron 22, 233–242 (1999).
Tsodyks, M.V., Skaggs, W.E., Sejnowski, T.J. & McNaughton, B.L. Population dynamics and theta rhythm phase precession of hippocampal place cell firing: a spiking neuron model. Hippocampus 6, 271–280 (1996).
Wallenstein, G.V. & Hasselmo, M.E. GABAergic modulation of hippocampal population activity: sequence learning, place field development, and the phase precession effect. J. Neurophysiol. 78, 393–408 (1997).
Kamondi, A., Acsady, L., Wang, X.J. & Buzsáki, G. Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: activity-dependent phase-precession of action potentials. Hippocampus 8, 244–261 (1998).
Bose, A., Booth, V. & Recce, M. A temporal mechanism for generating the phase precession of hippocampal place cells. J. Comput. Neurosci. 9, 5–30 (2000).
Bose, A. & Recce, M. Phase precession and phase-locking of hippocampal pyramidal cells. Hippocampus 11, 204–215 (2001).
Booth, V. & Bose, A. Neural mechanisms for generating rate and temporal codes in model CA3 pyramidal cells. J. Neurophysiol. 85, 2432–2445 (2001).
Yamaguchi, Y. A theory of hippocampal memory based on theta phase precession. Biol. Cybern. 89, 1–9 (2003).
Lengyel, M., Szatmary, Z. & Erdi, P. Dynamically detuned oscillations account for the coupled rate and temporal code of place cell firing. Hippocampus 13, 700–714 (2003).
Magee, J.C. A prominent role for intrinsic neuronal properties in temporal coding. Trends Neurosci. 26, 14–16 (2003).
Koene, R.A., Gorchetchnikov, A., Cannon, R.C. & Hasselmo, M.E. Modeling goal-directed spatial navigation in the rat based on physiological data from the hippocampal formation. Neural Net. 16, 577–584 (2003).
Sato, N. & Yamaguchi, Y. Memory encoding by theta phase precession in the hippocampal network. Neural Comput. 15, 2379–2397 (2003).
Ekstrom, A.D., Meltzer, J., McNaughton, B.L. & Barnes, C.A. NMDA receptor antagonism blocks experience-dependent expansion of hippocampal “place fields”. Neuron 30, 631–638 (2001).
Magee, J.C. Dendritic mechanisms of phase precession in hippocampal CA1 pyramidal neurons. J. Neurophysiol. 86, 528–532 (2001).
Buzsáki, G. & Czeh, G. Commissural and perforant path interactions in the rat hippocampus. Field potentials and unitary activity. Exp. Brain Res. 43, 429–438 (1981).
Buzsáki, G., Grastyan, E., Czopf, J., Kellenyi, L. & Prohaska, O. Changes in neuronal transmission in the rat hippocampus during behavior. Brain Res. 225, 235–247 (1981).
Douglas, R.M., McNaughton, B.L. & Goddard, G.V. Commissural inhibition and facilitation of granule cell discharge in fascia dentata. J. Comp. Neurol. 219, 285–294 (1983).
Buzsáki, G., Chen, L.S. & Gage, F.H. Spatial organization of physiological activity in the hippocampal region: relevance to memory formation. Prog. Brain Res. 83, 257–268 (1990).
Deadwyler, S.A., West, J.R., Cotman, C.W. & Lynch, G. Physiological studies of the reciprocal connections between the hippocampus and entorhinal cortex. Exp. Neurol. 49, 35–57 (1975).
Lisman, J.E. & Idiart, M.A. Storage of 7±2 short-term memories in oscillatory subcycles. Science 267, 1512–1515 (1995).
Gilden, D.L., Thornton, T. & Mallon, M.W. 1/f noise in human cognition. Science 267, 1837–1839 (1995).
Kudrimoti, H.S., Barnes, C.A. & McNaughton, B.L. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J. Neurosci. 19, 4090–4101 (1999).
Nadasdy, Z., Hirase, H., Czurkó, A., Csicsvari, J. & Buzsáki, G. Replay and time compression of recurring spike sequences in the hippocampus. J. Neurosci. 19, 9497–9507 (1999).
Lee, A.K. & Wilson, M.A. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36, 1183–1194 (2002).
Blum, K.I. & Abbott, L.F. A model of spatial map formation in the hippocampus of the rat. Neural Comput. 8, 85–93 (1996).
Mehta, M.R., Barnes, C.A. & McNaughton, B.L. Experience-dependent, asymmetric expansion of hippocampal place fields. Proc. Natl. Acad. Sci. USA 94, 8918–8921 (1997).
Buzsáki, G. Theta oscillations in the hippocampus. Neuron 33, 325–340 (2002).
Csicsvari, J., Hirase, H., Czurko, A. & Buzsáki, G. Reliability and state dependence of pyramidal cell-interneuron synapses in the hippocampus: an ensemble approach in the behaving rat. Neuron 21, 179–189 (1998).
Harris, K.D., Henze, D.A., Csicsvari, J., Hirase, H. & Buzsáki, G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J. Neurophysiol. 84, 401–414 (2000).
Harris, K.D., Hirase, H., Leinekugel, X., Henze, D.A. & Buzsáki, G. Temporal interaction between single spikes and complex spike bursts in hippocampal pyramidal cells. Neuron 32, 141–149 (2001).
Friedman, H.S. & Priebe, C.E. Estimating stimulus response latency. J. Neurosci. Methods 83, 185–194 (1998).
Acknowledgements
We thank M. Hasselmo, J. Lisman, J. Magee, M. Mehta, M. Tsodyks and Y. Yamaguchi for making the prediction of their models explicit after transient inactivation. We also thank K.D. Harris, A. Sirota and D.L. Buhl for assisting with data processing and L. Hazan, E. Pastalkova, S. Montgomery, S. Marguet and S. Royer for commenting on the manuscript. Supported by the National Institutes of Health (G.B.), the Human Frontier Science Program (M.B.Z.) and the French Defense Ministry (L.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Zugaro, M., Monconduit, L. & Buzsáki, G. Spike phase precession persists after transient intrahippocampal perturbation. Nat Neurosci 8, 67–71 (2005). https://doi.org/10.1038/nn1369
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1369
This article is cited by
-
Closed-loop brain stimulation augments fear extinction in male rats
Nature Communications (2023)
-
Acute silencing of hippocampal CA3 reveals a dominant role in place field responses
Nature Neuroscience (2019)
-
Recurrent circuits within medial entorhinal cortex superficial layers support grid cell firing
Nature Communications (2018)
-
The medial entorhinal cortex is necessary for temporal organization of hippocampal neuronal activity
Nature Neuroscience (2015)
-
Reversed theta sequences of hippocampal cell assemblies during backward travel
Nature Neuroscience (2014)