Abstract
The formation and disassembly of synapses in mature neuropil could provide a substrate to encode experience in the brain. Although there is evidence for postsynaptic spine dynamics in mature systems, contributions to circuit rearrangements by presynaptic terminals have remained unclear. We used hippocampal slice cultures from mice expressing spectral variants of green fluorescent protein (GFP) that are targeted to the membrane and/or synaptic vesicles in neuronal subsets to image identified presynaptic terminals. In mature tissues with no net change in synapse numbers, subpopulations of presynaptic terminals appeared and disappeared within 1–3 days. The three terminal types established by mossy fibers had distinct properties. High-frequency stimulation increased the fraction of dynamic terminals for 1–2 days, a process mediated by activation of AMPA receptors, protein kinase A (PKA) and protein synthesis. Thus, synaptic activity can make stable presynaptic terminals become dynamic, providing a candidate mechanism to convert experience into changes in network connectivity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bailey, C.H. & Kandel, E.R. Structural changes accompanying memory storage. Annu. Rev. Physiol. 55, 397–426 (1993).
Ziv, N.E. & Smith, S.J. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron 17, 91–102 (1996).
Marrs, G.S., Green, S.H. & Dailey, M.E. Rapid formation and remodeling of postsynaptic densities in developing dendrites. Nat. Neurosci. 4, 1006–1013 (2001).
Yuste, R. & Bonhoeffer, T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu. Rev. Neurosci. 24, 1071–1089 (2001).
Matus, A. Actin-based plasticity in dendritic spines. Science 290, 754–758 (2000).
Smart, F.M. & Halpain, S. Regulation of dendritic spine stability. Hippocampus 10, 542–554 (2000).
Lendvai, B., Stern, E.A., Chen, B. & Svoboda, K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature 404, 876–881 (2000).
Engert, F. & Bonhoeffer, T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399, 66–70 (1999).
Toni, N., Buchs, P.A., Nikonenko, I., Bron, C.R. & Muller, D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 402, 421–425 (1999).
Yankova, M., Hart, S.A. & Woolley, C.S. Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. Proc. Natl. Acad. Sci. USA 98, 3525–3530 (2001).
Luscher, C., Nicoll, R.A., Malenka, R.C. & Muller, D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat. Neurosci. 3, 545–550 (2000).
Cline, H.T. Dendritic arbor development and synaptogenesis. Curr. Opin. Neurobiol. 11, 118–126 (2001).
Stoppini, L., Buchs, P.A. & Muller, D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods 37, 173–182 (1991).
Gahwiler, B.H., Capogna, M., Debanne, D., McKinney, R.A. & Thompson, S.M. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 20, 471–477 (1997).
Henze, D.A., Urban, N.N. & Barrionuevo, G. The multifarious hippocampal mossy fiber pathway: a review. Neuroscience 98, 407–427 (2000).
Claiborne, B.J., Amaral, D.G. & Cowan, W.M. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J. Comp. Neurol. 246, 435–458 (1986).
Toth, K. & McBain, C.J. Target-specific expression of pre- and postsynaptic mechanisms. J. Physiol. 525, 41–51 (2000).
Acsady, L., Kamondi, A., Sik, A., Freund, T. & Buzsaki, G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J. Neurosci. 18, 3386–3403 (1998).
Toth, K., Suares, G., Lawrence, J.J., Philips-Tansey, E. & McBain, C.J. Differential mechanisms of transmission at three types of mossy fiber synapse. J. Neurosci. 20, 8279–8289 (2000).
Amaral, D.G. Synaptic extensions from the mossy fibers of the fascia dentata. Anat. Embryol. (Berl.) 155, 241–251 (1979).
Li, Z. & Murthy, V.N. Visualizing postendocytic traffic of synaptic vesicles at hippocampal synapses. Neuron 31, 593–605 (2001).
Ahmari, S.E., Buchanan, J. & Smith, S.J. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat. Neurosci. 3, 445–451 (2000).
Davis, G.W. & Bezprozvanny, I. Maintaining the stability of neural function: a homeostatic hypothesis. Annu. Rev. Physiol. 63, 847–869 (2001).
Dailey, M.E., Buchanan, J., Bergles, D.E. & Smith, S.J. Mossy fiber growth and synaptogenesis in rat hippocampal slices in vitro. J. Neurosci. 14, 1060–1078 (1994).
Ziv, N.E. & Garner, C.C. Principles of glutamatergic synapse formation: seeing the forest for the trees. Curr. Opin. Neurobiol. 11, 536–543 (2001).
Friedman, H.V., Bresler, T., Garner, C.C. & Ziv, N.E. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron 27, 57–69 (2000).
Mizuhashi, S., Nishiyama, N., Matsuki, N. & Ikegaya, Y. Cyclic nucleotide-mediated regulation of hippocampal mossy fiber development: a target-specific guidance. J. Neurosci. 21, 6181–6194 (2001).
Baranes, D. et al. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron 21, 813–825 (1998).
Hatada, Y., Wu, F., Sun, Z.Y., Schacher, S. & Goldberg, D.J. Presynaptic morphological changes associated with long-term synaptic facilitation are triggered by actin polymerization at preexisting varicosities. J. Neurosci. 20, RC82 (2000).
Bozdagi, O., Shan, W., Tanaka, H., Benson, D.L. & Huntley, G.W. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron 28, 245–259 (2000).
Ma, L., Zablow, L., Kandel, E.R. & Siegelbaum, S.A. Cyclic AMP induces functional presynaptic boutons in hippocampal CA3-CA1 neuronal cultures. Nat. Neurosci. 2, 24–30 (1999).
Patterson, S.L. et al. Some forms of cAMP-mediated long-lasting potentiation are associated with release of BDNF and nuclear translocation of phospho-MAP kinase. Neuron 32, 123–140 (2001).
Horch, H.W., Kruttgen, A., Portbury, S.D. & Katz, L.C. Destabilization of cortical dendrites and spines by BDNF. Neuron 23, 353–364 (1999).
Poo, M.M. Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2, 24–32 (2001).
Castillo, P.E., Schoch, S., Schmitz, F., Sudhof, T.C. & Malenka, R.C. RIM1α is required for presynaptic long-term potentiation. Nature 415, 327–330 (2002).
Cremer, H. et al. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature 367, 455–459 (1994).
Antonova, I. et al. Rapid increase in clusters of presynaptic proteins at onset of long-lasting potentiation. Science 294, 1547–1550 (2001).
Nimchinsky, E.A., Sabatini, B.L. & Svoboda, K. Structure and function of dendritic spines. Annu. Rev. Physiol. 64, 313–353 (2002).
Dailey, M.E. & Smith, S.J. The dynamics of dendritic structure in developing hippocampal slices. J. Neurosci. 16, 2983–2994 (1996).
Bastmeyer, M. & O'Leary, D.D. Dynamics of target recognition by interstitial axon branching along developing cortical axons. J. Neurosci. 16, 1450–1459 (1996).
Pennetta, G., Hiesinger, P., Fabian-Fine, R., Meinertzhagen, I. & Bellen, H. Drosophila VAP-33A directs bouton formation at neuromuscular junctions in a dosage-dependent manner. Neuron 35, 291 (2002).
Lichtman, J.W. & Colman, H. Synapse elimination and indelible memory. Neuron 25, 269–278 (2000).
Shi, S., Hayashi, Y., Esteban, J.A. & Malinow, R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105, 331–343 (2001).
Okabe, S., Kim, H.D., Miwa, A., Kuriu, T. & Okado, H. Continual remodeling of postsynaptic density and its regulation by synaptic activity. Nat. Neurosci. 2, 804–811 (1999).
Pun, S. et al. An intrinsic distinction in neuromuscular junction assembly and maintenance in different skeletal muscles. Neuron 34, 357–370 (2002).
Caroni, P. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J. Neurosci. Methods 71, 3–9 (1997).
Wiederkehr, A., Staple, J. & Caroni, P. The motility-associated proteins GAP-43, MARCKS, and CAP-23 share unique targeting and surface activity-inducing properties. Exp. Cell Res. 236, 103–116 (1997).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Acknowledgements
We thank the members of the Caroni group for discussions, Y. Barde and D. Muller for comments on the manuscript, M. Moosmayer for introducing us to the slice culture technique, the Matus lab for technical help, L. Xu for help with electrophysiology and I. Dodevsky for help with quantifications. We are very grateful to M. Abegg and M. Scanziani for help with the electrophysiology experiments. S.A. was supported by the Swiss National Science Foundation and the Kanton Basel-Stadt. The Friedrich Miescher Institute is a branch of the Novartis Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1.
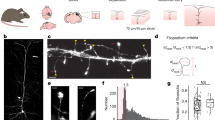
Hippocampal slice cultures from Thy1-mGFPs mice allow the visualization of the terminal arborization of subsets of Dentate Gyrus (DG) granule cells. Images are projections of confocal stacks spanning 51.15 (large panel), 33.17 (left bottom panel), and 11.04 μm (right bottom panel) in the z-dimension. Large panel shows at low magnification the DG-CA3 area of the hippocampus. Note mossy fiber axons originating in the granule cell layer (boxed region 1), exiting the hilus (H), and coursing in the CA3 region (arrows), where their terminals were imaged. Left bottom panel is a high magnification image of the boxed region 1. Note granule cells bodies (asterisks), basal dendrites and the initial part of MF axons (arrows). The right bottom panel is a higher magnification confocal stack through the boxed region 2. Note the relatively few MF that allow high resolution imaging of their terminals. Three examples of LMTs are indicated by the arrowheads. Arrow bar is 130 μm in the large panel and 45 μm in the two small ones. (JPG 78 kb)
Supplementary Fig. 2.
Visualization of terminal arborization with mGFP, and of presynaptic terminals with spGFP. Imaging of acute whole mount gluteus muscle preparations from Thy1-mGFPmu and Thy1-spGFPmu mice. Neuromuscular junctions were counterstained with α-Bungarotoxin to visualize postsynaptic Acetylcholine receptors (αBtx; merged images). The characteristic arrangement of pre- and postsynaptic elements at the neuromuscular junction allows for a detailed validation of the subcellular compartment markers used in this study. The mGFP membrane marker visualizes motor axons and the details of their arborization at the neuromuscular junction, whereas spGFP selectively visualizes presynaptic terminals (see also21). The inserts show higher magnification views of individual neuromuscular junctions. Bar: 50 μm. (JPG 27 kb)
Supplementary Fig. 3.
Characteristic presynaptic patterns visualized by spGFP in slices from Thy1-spGFPm mice. (a-k): un-processed fluorescence images of CA1 (a, c-e) and Dentate Gyrus (DG)-CA3 (b, f-k) regions from living hippocampal slice cultures. (a) and (b) are low magnification examples from CA1 pyramidal layer and CA3b regions shown at the same magnification. Note punctuate synaptic pattern of expression of spGFP; larger spGFP clusters in CA3 presumably correspond to MF terminals (arrows). Asterisks mark cell bodies. An example of region CA1 stratum radiatum at low magnification is shown in (c). In (e) a high magnification of the boxed region from (d) is displayed. Note clear puncta in CA1 stratum radiatum (arrows). Low magnification of the DG-CA3 border is shown in (f). The infrapyramidal (INFRA) and suprapyramidal (SUPRA) mossy fiber terminal layers are indicated. A low magnification image of the DG Molecular Layer (ML)- Granule cell Layer (GCL)- Hilus (H) boundaries is shown in (g). Note larger spGFP clusters in the hilus, where MF terminals are also located. A high magnification image of the boxed region in (h) is shown in (i). Note the prominent accumulations of spGFP, corresponding to the large MF terminals (arrows); these are never observed in region CA1 (see (e) for a comparison at the same magnification). In (k) the high magnification picture from boxed region in (j), shows presynaptic puncta on a CA3 pyramidal neuron dendrite (evident from background signal) (arrows) and cell body (arrowhead). Scale bars: (a, b) 45, (c) 70, (d) 30, (e, i) 8, (f) 145, (g) 65, (h) 55, (j) 12, (k) 6 μm. (JPG 130 kb)
Supplementary Fig. 4.
Remodeling of mossy fiber axons in young, but not mature hippocampal slice cultures. Note the dramatic change in axonal branching and growth pattern over 1 day in the representative example of young tissue (9 DIV, Days In Vitro, arrowheads, top panels). Arrows indicate LMTfil originating from LMT (asterisks). Ax, axon. Bar is 6 μm. (JPG 26 kb)
Supplementary Fig. 5.
LMT undergo spatial rearrangement in shape, but do not change their numbers or position over many hours in mature hippocampal slice cultures. Top panels show an example of confocal time-lapse imaging on two LMTs (asterisks) in a Thy1-mGFPs slice. Arrow points to an LMTfil. Each of the 3 un-processed panels is a projection of 63 images spanning 11 μm in the z-dimension. Bottom panels are frames from a representative confocal series from a Thy1-spGFPm slice. Each panel represents a projection of 36 images spanning 6.3 μm in the z-dimension of a representative field containing the LMT indicated by the asterisk in its entirety. Note shape alterations over 18h period. The smaller panels show the same LMT (asterisk) from different angles. Bar is 3 μm for top panels and 2 μm for bottom ones. (JPG 32 kb)
Supplementary Fig. 6.
Mossy fibers are characterized by a dynamic activity at axonal filopodia and varicosities in the adult hippocampus ex vivo. (a, b) The panels are fluorescent un-processed pictures of mossy fibers from a 120d whole mount hippocampal acute preparation. (a) shows at low magnification a mossy fiber coursing from bottom left to top right. The three panels at the bottom represent the boxed region imaged over 8 minutes. Note the retraction of a filopodium indicated by the arrow. (b) The panel shows a low magnification of a MF bearing an epV (boxed region). The same epV is shown in the three bottom panels imaged over 10 min. Note a rapid phase of filopodial extension-retraction from the epV, indicated by the arrow. Bar is 3.7 in (a) and 1.5 μm in (b). (JPG 24 kb)
Supplementary Fig. 7.
Propidium Iodide (PI) assay for cell death in long term hippocampal slice cultures. Control and treated slices were analyzed for the presence of cell death (see Methods) 1 and 2 days after treatments. All hippocampal slices are positioned in the same orientation, with the CA3 region on the right. (a) Positive control: a slice left for 4 days without nutrients and O2 is degenerating. Note in (b) at high magnification the fluorescent nuclei (arrows). (c) Control slice, 1 month-old. A broken glass electrode was used to kill cells locally (arrow), and investigate their removal kinetics. After 2 days they were still detectable indicating that PI incorporation is an appropriate tool to investigate the possible toxicity of our treatments. (d) Further example of untreated 1month slice culture. Note variability, but relative paucity in the number of dead cells, when compared to (c). (e) Example of a slice kept for 3 months in vitro, without further treatments. (f) Example of a 34 day old AMPA+AP5 treated slice, 2 days after application of the drugs. (g) 1 month slice stimulated with 100 Hz protocol and assayed for PI incorporation 2 days later. (h) Example of the digital mask applied in this case to the raw image in (c), to count number of cells with Image software. (i) Quantification of dead cells in 1month and 3 month cultures. Data from each condition are mean±SD from 4-6 slices, 3 independent experiments. There was no significant increase in the number of dead cells 1 day after the treatments (P = 0.7 and 0.8 for AMPA + AP5 and 100 Hz respectively, versus control group). Same results were obtained for Glutamate, TTX, BDNF and cAMP under the conditions used in our experiments. Moreover, all the drugs were tested at higher concentrations and for longer times in cell death assays, and used at lower concentrations and for shorter times than those that proved to be safe for the presynaptic dynamics experiments. (j-l) Simultaneous AMPA-R and cAMP-dependent pathway activation leads to extensive cell death in hippocampal slice cultures. We obtained the same results with high doses of AMPA. (j) 1 day after treatment, (k) 2 days after treatment. (l) Degenerating MF in the slice shown in (j). Arrows point to beaded areas indicating a degenerating axon. (JPG 50 kb)
Supplementary Fig. 8.
Representative unprocessed data from mGFP imaging sessions of slice cultures used in this study, exhibit high signal to noise ratios. (see also Suppl. Figs. 1, 3, 4, 5 for other examples of unprocessed data). The processed picture representing a MF axon, was slightly contrasted in Photoshop. (JPG 12 kb)
Supplementary Video 1.
There are 2 distinct classes of Thy1-spGFPm expressing clusters in mature slice cultures. The video illustrates the difference between small clusters (size range of transport packets, arrows) and larger ones (> 1.5 μm) in their dynamic behavior over a few minutes. Clusters in the size range of transport packets (0.3-0.6 μm, long axis; 0.2-0.4 μm, short axis) were seen moving rapidly back and forth along the axon (arrows). Larger clusters (> 1.5) did not move over minutes. Three stable clusters are indicated by the arrowheads. Frames were captured at 10 sec intervals, and the video sequence is shown at 15 frames/second. (MOV 410 kb)
Supplementary Video 2.
Mature MF are characterized by foci of dynamic activity. The video shows a MF axon from a Thy1-mGFPs slice (40div) imaged over a few minutes. The arrow points to an epV, where protrusive activity (rapid filopodia extension-retraction events) is evident. On the same axon an LMT is out of focus (asterisk), and 2 of its filopodia are marked by the arrowheads. Frames were captured at 10 sec intervals; the video has been time-compressed, and is shown at 15 frames/second. (MOV 683 kb)
Supplementary Video 3.
Young MF are characterized by more frequent and prominent dynamic activity than mature ones. The video shows a young MF axon from a Thy1-mGFPs slice imaged over a few minutes (same slice culture as Suppl. Video 2). The corresponding granule cell exhibited the characteristic features of young granule cells: including the location in the DG GCL, a granular shape of cell body, one basal dendrite, no real spines on the dendrite but highly motile filopodia-like spines and highly motile growth cone filopodia at the end of axonal branches. Note an epV (arrow), where multiple events of filopodial extension-retraction are visible. A dynamic filopodium is evident towards the left (arrowhead). Frames were captured at 10 sec intervals, and the video is shown at 15 frames/second. (MOV 259 kb)
Rights and permissions
About this article
Cite this article
De Paola, V., Arber, S. & Caroni, P. AMPA receptors regulate dynamic equilibrium of presynaptic terminals in mature hippocampal networks. Nat Neurosci 6, 491–500 (2003). https://doi.org/10.1038/nn1046
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1046
This article is cited by
-
Pharmacological ablation of astrocytes reduces Aβ degradation and synaptic connectivity in an ex vivo model of Alzheimer’s disease
Journal of Neuroinflammation (2021)
-
In vivo imaging of injured cortical axons reveals a rapid onset form of Wallerian degeneration
BMC Biology (2020)
-
Regional transcriptome analysis of AMPA and GABAA receptor subunit expression generates E/I signatures of the human brain
Scientific Reports (2020)
-
APC2 controls dendrite development by promoting microtubule dynamics
Nature Communications (2018)
-
Deep tissue analysis of distal aqueous drainage structures and contractile features
Scientific Reports (2017)