Abstract
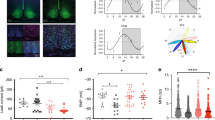
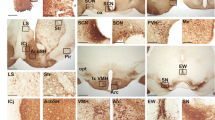
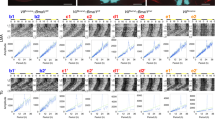
Phospholipase C β4 (PLCβ4) is expressed in the suprachiasmatic nucleus (SCN), as well as in tissues in the circadian entrainment pathway, including the retina and the lateral geniculate nucleus in the mouse1,2. Using PLCβ4−/− mice, we previously reported that PLCβ4 is coupled to metabotropic glutamate receptors (mGluRs)3, which regulate the ionotropic glutamate responsiveness of SCN neurons, and thus were proposed to be involved in photic entrainment4. We show here that the group-I mGluR–PLCβ4 signaling pathway is involved in translating circadian oscillations of the molecular clock into rhythmic outputs of SCN neurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ikeda, M. et al. Neuroreport 11, 907–912 (2000).
Jiang, H. et al. Proc. Natl. Acad. Sci. USA 93, 14598–14601 (1996).
Kim, D. et al. Nature 389, 290–293 (1997).
Haak, L.L. J. Neurophysiol. 81, 1308–13017 (1999).
Klein, D.C., Moore, R.C. & Reppert, S.M. Suprachiasmatic Nucleus: The Mind's Clock (Oxford Univ. Press, New York, 1991).
Dunlap, J.C. Cell 96, 271–290 (1999).
Reppert, S.M. & Weaver, D.R. Annu. Rev. Physiol. 63, 647–676 (2001).
Shinohara, K., Honma, S., Katsuno, Y., Abe, H. & Honma, K. Neuroreport 9, 137–140 (1998).
Shinohara, K., Honma, S., Katsuno, Y. & Honma, K. Neurosci. Res. 36, 245–250 (2000).
Gillette, M.U. & Reppert, S.M. Brain Res. Bull. 19, 135–139 (1987).
Cheng, M.Y. et al. Nature 417, 405–410 (2002).
Kramer, A. et al. Science 294, 2511–2515 (2001).
Acknowledgements
This work was supported by the National Creative Research Initiative (CRI) Program of Korea (H-S.S.) and the Brain Korea 21 Project in 2002 (Y.I.K.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1.
Mean escape latencies for animals in the visible-platform version of the Morris water task. Wild-type and PLCβ4-/- mice were trained to navigated to a randomly located visible platform. The platform was rendered visible by attaching a mast topped with a circular black cylinder. Each animal was first trained to climb on the platform and given a 15 s practice swim to ensure that all animals could swim. On each trial, a subject was allowed to search the pool for a maximum of 60 s. Once a subject found the platform, it was allowed to remain there for 30 s. Animals were given 8 trials a day, in blocks of 2 trials. Performance improved for each genotype (one-way ANOVA, F3,16 = 5.64, P < 0.05 in wild-type mice; F3,16 = 6.89, P < 0.01 in PLC4-/- mice) and there was no significant difference between wild-type and mutant mice (two-way ANOVA, F1,8 = 0.05, P > 0.1). (PDF 56 kb)
Supplementary Fig. 2.
Fourier analysis and periodogram of behavioral activity rhythms. Analyses are for the mice whose actograms are shown in Fig. 1. The analyses shown were conducted on the last 7-day segment in LD and the first and last 7-day segments in DD. The amplitude of any rhythm (y-axis) is plotted against the number of cycles per day for (a) fast Fourier transform and length of period for (b) chi-square periodogram. Note the changes in y-axis between panels, indicative of the loss of rhythm amplitude in DD. (PDF 291 kb)
Supplementary Fig. 3.
Persistence of circadian rhythm under constant darkness. Data were analyzed as described in Supplementary Methods online. For each mouse, the ratio of the relative power value for data blocks in DD to that in LD was obtained and used as a measure of rhythm persistence in wild-type (solid bars) and PLCβ4-/- mice (open bars). A ratio value of 1 means that circadian rhythm in DD is as persistent as it is in LD. Values are expressed as mean ± s.e.m. Two-way ANOVA revealed significant effects of genotype (F1,25 = 33.97, P < 0.0001) and a significant interaction between genotype and time (F3,100 = 3.69, P < 0.05). The amount of total activity differed between mice of F1 and 129 genetic backgrounds, and between wild-type and mutant mice. However, there was no significant difference in the activities between LD and DD for a given group of mice. Therefore, loss of circadian rhythm in mutant mice during DD was not due to a change in total activity compared with LD. (PDF 84 kb)
Supplementary Fig. 4.
Expression of Bmal1 in the SCN of wild-type and PLCβ4-/- mice on the third day in constant darkness. Peak values observed in wild-type mice at CT 16 were used as the reference to obtain relative RNA abundance of Bmal1 at each time point. Values are expressed as mean ± s.e.m. Two-way ANOVA revealed a significant effect of time but not of genotype. One-way ANOVA revealed significant rhythms in each genotype (F5,13 = 4.03, P < 0.05 in wild-type; F5,12 = 3.20, P < 0.05 in PLCβ4-/- mice). Representative autoradiograms are shown. (PDF 82 kb)
Rights and permissions
About this article
Cite this article
Park, D., Lee, S., Jun, K. et al. Translation of clock rhythmicity into neural firing in suprachiasmatic nucleus requires mGluR–PLCβ4 signaling. Nat Neurosci 6, 337–338 (2003). https://doi.org/10.1038/nn1033
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1033
This article is cited by
-
Retina-attached slice recording reveals light-triggered tonic GABA signaling in suprachiasmatic nucleus
Molecular Brain (2021)
-
Phospholipase C beta 4 in mouse hepatocytes: Rhythmic expression and cellular distribution
Comparative Hepatology (2008)