Abstract
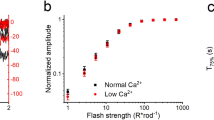
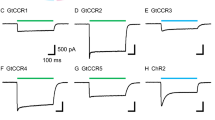
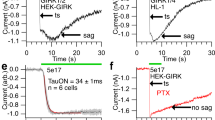
All cellular signaling pathways currently known to elevate cGMP involve the activation of a guanylyl cyclase to synthesize cGMP. Here we describe an exception to this rule. In the vertebrate parietal eye, the photoreceptors depolarize to light under dark-adapted conditions, unlike rods and cones but like most invertebrate photoreceptors. We report that the signaling pathway for this response involves a rise in intracellular cGMP resulting from an inhibition of the phosphodiesterase that hydrolyzes cGMP. Furthermore, this phosphodiesterase is driven by an active G protein in darkness. These results indicate an antagonistic control of the phosphodiesterase by two G proteins, analogous to the Gs/Gi control of adenylyl cyclase. Our findings demonstrate an unusual phototransduction mechanism and at the same time indicate that signaling involving cyclic nucleotides is more elaborate than previously known.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Stryer, L. Cyclic GMP cascade of vision. Annu. Rev. Neurosci. 9, 87–119 (1986).
Lagnado, L. & Baylor, D. A. Signal flow in visual transduction. Neuron 8, 995–1002 (1992).
Pugh, E. N. Jr & Lamb, T. D. Amplification and kinetics of the activation steps in phototransduction. Biochim. Biophys. Acta 1141, 111–149 (1993).
Yarfitz, S. & Hurley, J. B. Transduction mechanism of vertebrate and invertebrate photoreceptors. J. Biol. Chem. 269, 14329–14332 (1994).
Yau, K. -W. & Baylor, D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu. Rev. Neurosci. 12, 289–327 (1989).
Eakin, R. M. in The Third Eye (University of California Press, Berkeley, 1973).
Solessio, E. & Engbretson, G. A. Antagonistic chromatic mechanisms in photoreceptors of the parietal eye of lizards. Nature 364, 442–445 (1993).
Hardie, R. C. & Minke, B. Phosphoinositide-mediated phototransduction in Drosophila photoreceptors: the role of Ca2+ and trp. Cell Calcium 18, 256–274 (1995).
Ranganathan, R., Malicki, D. M. & Zuker, C. S. Signal transduction in Drosophila photoreceptor. Annu. Rev. Neurosci. 18, 283–317 (1995).
Shin, J., Richard, E. A. & Lisman, J. E. Ca2+ is an obligatory intermediate in the excitation cascade of limulus photoreceptors. Neuron 11, 845–855 (1993).
Finn, J. T., Solessio, E. C. & Yau, K. -W. A cGMP-gated cation channel in depolarizing photoreceptors of the lizard parietal eye. Nature 385, 815–819 (1997).
Horn, R. & Marty, A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J. Gen. Physiol. 92, 145–159 (1988).
Koch, K. -W. & Kaupp, U. B. Cyclic GMP directly regulates a cation conductance in membranes of bovine rods by a cooperative mechanism. J. Biol. Chem. 260, 6788–6800 (1985).
Nicol, G. D., Schnetkamp, P. P., Saimi, Y., Cragoe, E. J. Jr & Bownds, M. D. A derivative of amiloride blocks both the light-regulated and cyclic GMP-regulated conductances in rod photoreceptors. J. Gen. Physiol. 90, 651–669 (1987).
Beavo, J. A. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol. Rev. 75, 725–748 (1995).
Gillespie, P. G. & Beavo, J. A. Inhibition and stimulation of photoreceptor phosphodiesterases by dipyridamole and M&B 22,948. Mol. Pharmacol. 36, 773–781 (1989).
Gilman, A. G. G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 56, 615–649 (1987).
Bigay, J., Deterre, P., Pfister, C. & Chabre, M. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 191, 181–185 (1985).
Pugh, E. N. Jr, Lisman, J. & Payne, R. Strange case of the third eye. Nature 364, 389–390 (1993).
Sunahara, R. K., Dessauer, C. W. & Gilman, A. G. Complexity and diversity of mammalian adenylyl cyclases. Annu. Rev. Pharmacol. Toxicol. 36, 461–480 (1996).
Ui, M. & Katada, T. Bacterial toxins as probe for receptor-Gi coupling. Adv. Second Messenger Phosphoprotein Res. 24, 63–69 (1990).
Birnbaumer, L. G proteins in signal transduction. Annu. Rev. Pharmacol. Toxicol. 30, 675–705 (1990).
Simon, M. I., Strathmann, M. P. & Gautam, N. Diversity of G proteins in signal transduction. Science 252, 802–808 (1991).
Drewett, J. G. & Garbers, D. L. The family of guanylyl cyclase receptors and their ligands. Endocr. Rev. 15, 135–162 (1994).
Wedel, B. J. & Garbers, D. L. New insights on the functions of the guanylyl cyclase receptors. FEBS Lett. 410, 29–33 (1997).
Zhang, J. & Snyder, S. H. Nitric oxide in the nervous system. Annu. Rev. Pharmacol. Toxicol. 35, 213–233 (1995).
Gudermann, T., Schoneberg, T. & Schultz, G. Functional and structural complexity of signal transduction via G-protein-coupled receptors. Annu. Rev. Neurosci. 20, 399–427 (1997).
Gomez, M. & Nasi, E. Activation of light-dependent K+ channels in ciliary invertebrate photoreceptors involves cGMP but not the IP3/Ca2+ cascade. Neuron 15, 607–618 (1995).
Li, Y., Hanf, R., Otero, A. S., Fischmeister, R. & Szabo, G. Differential effects of pertussis toxin on the muscarinic regulation of Ca2+ and K+ currents in frog cardiac myocytes. J. Gen. Physiol. 104, 941–959 (1994).
Zong, X. & Lux, H. D. Augmentation of calcium channel currents in response to G protein activation by GTP gamma S in chick sensory neurons. J. Neurosci. 14, 4847–4853 (1994).
Acknowledgements
We thank Roger C. Hardie and Yiannis Koutalos for suggestions about experiments, and Joe Beavo, Jackie D. Corbin, Peter N. Devreotes, Vincent Manganiello, Enrico Nasi, Carol Parent, and W. Joe Thompson for discussions. Robert D. Barber, Peter G. Gillespie, Maria E. Grunwald, Dietmar Krautwurst and Haining Zhong provided comments on the manuscript. We also thank Eric Lasater for his generosity in making this collaboration possible. The L- cis -diltiazem was a gift from Tanabe Seyaku Co. (Osaka, Japan). This work was supported by a grant from the U.S. National Eye Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiong, WH., Solessio, E. & Yau, KW. An unusual cGMP pathway underlying depolarizing light response of the vertebrate parietal-eye photoreceptor. Nat Neurosci 1, 359–365 (1998). https://doi.org/10.1038/nn0998_359
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nn0998_359
This article is cited by
-
C. elegans phototransduction requires a G protein–dependent cGMP pathway and a taste receptor homolog
Nature Neuroscience (2010)