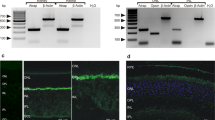
Abstract
To determine whether the concentrations of calcium-binding proteins present in some neurons and sensory cells are sufficient to influence presynaptic calcium signaling, we studied the predominant calcium-binding protein in a class of sensory hair cells in the frog ear. Based on antibody affinity and molecular weight, we identified this protein as calretinin. We measured its cytoplasmic concentration to be ∼1.2 mM, sufficient to bind ∼6 mM Ca2+. Calcium signaling was altered when the diffusible cytoplasmic components were replaced by an intracellular solution lacking any fast calcium buffer, and was restored by the addition of 1.2 mM exogenous calretinin to the intracellular solution. We conclude that calretinin, when present at millimolar concentration, can serve as a diffusionally mobile calcium buffer/transporter capable of regulating calcium signaling over nanometer distances at presynaptic sites.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Baimbridge, K. G., Celio, M. R. & Rogers, J. H. Calcium-binding proteins in the nervous system. Trends Neurosci. 15, 303–308 (1992).
Billing-Marczak, K. & Kuznicki J. Calretinin—sensor or buffer—function still unclear. Pol. J. Pharmacol. 51, 173–178 (1999).
Lephart, E. D. & Watson, M. A. Maternal separation: hypothalamic-preoptic area and hippocampal calbindin-D28K and calretinin in male and female infantile rats. Neurosci. Lett. 267, 41– 44 (1999).
Airaksinen, M. S., Thoenen, H. & Meyer, M. Vulnerability of midbrain dopaminergic neurons in calbindin-D28k-deficient mice: lack of evidence for a neuroprotective role of endogenous calbindin in MPTP-treated and weaver mice. Eur. J. Neurosci. 9, 120–127 (1997).
Kuznicki J., Isaacs, K. R. & Jacobowitz, D. M. The expression of calretinin in transfected PC12 cells provides no protection against Ca2+-overload or trophic factor deprivation. Biochim. Biophys. Acta 1313, 194–200 (1996).
Rogers, J. H. Calretinin: a gene for a novel calcium-binding protein expressed principally in neurons. J. Cell Biol. 105, 1343– 1353 (1987).
Roberts, W. M. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J. Neurosci. 14, 3246– 3262 (1994).
Chabbert, C. H. Heterogeneity of hair cells in the bullfrog sacculus. Pflugers Arch. 435, 82–90 ( 1997).
Sabatini, B. L. & Regehr, W.G . Timing of neurotransmission at fast synapses in the mammalian brain. Nature 384 , 170–172 (1996).
Roberts, W. M., Jacobs, R. A. & Hudspeth, A. J. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J. Neurosci. 10, 3664– 3684 (1990).
Neher, E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron 20, 389–399 (1998).
Lenzi, D., Runyeon, J. W., Crum, J., Ellisman, M. H. & Roberts, W. M. Synaptic vesicle populations in saccular hair cells reconstructed by electron tomography. J. Neurosci. 19, 119–132 (1999).
Wu, Y.-C., Tucker, T. & Fettiplace, R. A theoretical study of calcium microdomains in turtle hair cells. Biophys. J. 71, 2256– 2275 (1996).
Naraghi, M. & Neher, E. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J. Neurosci. 17, 6961–6973 (1997).
Adler, E. M., Augustine, G. J., Duffy, S. N. & Charlton, M. P. Alien calcium chelators attenuate neurotransmitter release at the squid giant synapse J. Neurosci. 11, 1496– 1507 (1991).
Roberts, W. M. Spatial calcium buffering in frog saccular hair cells. Nature 363, 74–76 (1993).
Ouanounou, A. et al. Accumulation and extrusion of permeant Ca2+ chelators in attenuation of synaptic transmission at hippocampal CA1 neurons . Neuroscience 75, 99–109 (1996).
Hall, J. D., Betarbet, S. & Jaramillo, F. Endogenous buffers limit the spread of free calcium in hair cells. Biophys. J. 73, 1243– 1252 (1997).
Ohana, O. & Sakmann, B. Transmitter release modulation in nerve terminals of rat neocortical pyramidal cells by intracellular calcium buffers. J. Physiol. (Lond.) 513, 135– 148 (1998).
Ricci, A. J., Wu, Y.-C. & Fettiplace, R. The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. J. Neurosci. 18, 8261–8277 (1998).
Moffat, A. J. M. & Capranica, R. R. Auditory sensitivity of the saccule in the American toad (Bufo americanus) J. Comp Physiol. A 105, 1–8 (1976).
Oberholtzer, J. C., Buettger, C., Summers, M. C. & Matschinsky, F. M. The 28-kDa calbindin-D is a major calcium-binding protein in the basilar papilla of the chick. Proc. Natl. Acad. Sci. USA 85, 3387–3390 (1988).
Tucker, T. R. & Fettiplace, R. Monitoring calcium in turtle hair cells with a calcium-activated potassium channel. J. Physiol. (Lond.) 494, 613–626 ( 1996).
Pinol, M. R. et al. Poly- and monoclonal antibodies against recombinant rat brain calbindin D-28K were produced to map its selective distribution in the central nervous system. J. Neurochem. 54, 1827– 1833 (1990).
Gona, A. G., Pendurthi, T. K., Al-Rabiai, S., Gona, O. & Christakos, S. Immunocytochemical localization and immunological characterization of vitamin D-dependent calcium-binding protein in the bullfrog cerebellum. Brain Behav. Evol. 29, 176–183 (1986).
Celio, M. R. et al. Monoclonal antibodies directed against the calcium binding protein Calbindin D-28k. Cell Calcium 11, 599–602 (1990).
Armstrong, C. E. & Roberts, W. M. Electrical properties of frog saccular hair cells: distortion by enzymatic dissociation . J. Neurosci. 18, 2962– 2973 (1998).
Schwaller, B., Durussel, I., Jermann, D, Herrmann, B. & Cox, J. A. Comparison of the Ca2+-binding properties of human recombinant calretinin-22k and calretinin. J. Biol. Chem. 272, 29663–29671 ( 1997).
Stevens, J. & Rogers, J. H. Chick calretinin: purification, composition, and metal binding activity of native and recombinant forms. Protein Expression Purification 9, 171– 181 (1997).
Dong, W.-J., Rosenfeld, S. S., Wang, C.-K., Gordon, A. M. & Cheung, H. C. Kinetic studies of calcium binding to the regulatory site of troponin C from cardiac muscle. J. Biol. Chem. 271, 688–694 ( 1996).
Winsky, L. & Kuznicki, J. Antibody recognition of calcium-binding proteins depends on their calcium-binding status. J. Neurochem. 66, 764–771 ( 1996).
Lumpkin, E. A. & Hudspeth, A. J. Detection of Ca2+ entry through mechanosensitive channels localizes the site of mechanoelectrical transduction in hair cells. Proc. Natl. Acad. Sci. USA 92, 10297–10301 (1995).
Gillespie, P. G. & Gillespie, K. H. Improved electrophoresis and transfer of picogram amounts of protein with hemoglobin . Anal. Biochem. 246, 239– 245 (1997).
Shepherd, G. M. G., Barres, B. A. & Corey, D. P. Bundle blot purification and initial protein characterization of hair cell stereocilia. Proc. Natl. Acad. Sci. USA 86, 4973–4977 (1989).
Acknowledgements
We thank M. Celio for providing antibodies and recombinant protein, L. Hansen, P. Gillespie and D. Rivers and for technical advice and D. Lenzi for commenting on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Edmonds, B., Reyes, R., Schwaller, B. et al. Calretinin modifies presynaptic calcium signaling in frog saccular hair cells. Nat Neurosci 3, 786–790 (2000). https://doi.org/10.1038/77687
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/77687
This article is cited by
-
The Piezo channel is a mechano-sensitive complex component in the mammalian inner ear hair cell
Nature Communications (2024)
-
Regional distribution of calretinin and calbindin-D28k expression in the brain of the urodele amphibian Pleurodeles waltl during embryonic and larval development
Brain Structure and Function (2013)
-
Nanodomain coupling between Ca2+ channels and sensors of exocytosis at fast mammalian synapses
Nature Reviews Neuroscience (2012)
-
Effects of Calretinin on Ca2+ Signals in Cerebellar Granule Cells: Implications of Cooperative Ca2+ Binding
The Cerebellum (2012)
-
Exocytosis in the Frog Amphibian Papilla
Journal of the Association for Research in Otolaryngology (2012)