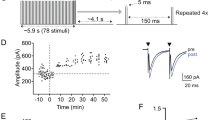
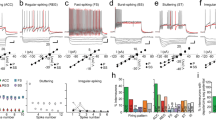
Abstract
Prolonged low-frequency stimulation of excitatory afferents to basolateral amygdala neurons results in enduring enhancement of excitatory synaptic responses. The induction of this form of synaptic plasticity is eliminated by selective antagonists of GluR5 kainate receptors and can be mimicked by the GluR5 agonist ATPA. Kainate receptor-mediated synaptic facilitation generalizes to include inactive afferent synapses on the target neurons, and therefore contrasts with other types of activity-dependent enduring synaptic facilitation that are input-pathway specific. Such heterosynaptic spread of synaptic facilitation could account for adaptive and pathological expansion in the set of critical internal and external stimuli that trigger amygdala-dependent behavioral responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Malenka, R. C. Synaptic plasticity in the hippocampus: LTP and LTD. Cell 78, 535–538 (1994).
Kirkwood, A. et al. Common forms of synaptic plasticity in the hippocampus and neocortex in vitro. Science 260, 1518–1521 (1993).
Maren, S. Synaptic transmission and plasticity in the amygdala. An emerging physiology of fear conditioning circuits. Mol. Neurobiol. 13, 1–22 (1996).
Li, H., Weiss, S. R., Chuang, D. M., Post, R. M. & Rogawski, M. A. Bidirectional synaptic plasticity in the rat basolateral amygdala: characterization of an activity-dependent switch sensitive to the presynaptic metabotropic glutamate receptor antagonist 2S-α-ethylglutamic acid. J. Neurosci. 18, 1662–1670 (1998).
McDonald, A. J. & Mascagni, F. Projections of the lateral entorhinal cortex to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 77, 445–459 (1997).
Mascagni, F., McDonald, A. J. & Coleman, J. R. Corticoamygdaloid and corticocortical projections of the rat temporal cortex: a Phaseolus vulgaris leucoagglutinin study. Neuroscience 57, 697–715 (1993).
Davis, M., Rainnie, D. & Cassell, M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 17, 208–214 (1994).
Li, H. & Rogawski, M. A. GluR5 kainate receptor mediated synaptic transmission in rat basolateral amygdala in vitro. Neuropharmacology 37, 1279–1286 (1998).
Paternain, A. V., Morales, M. & Lerma, J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron 14, 185–189 (1995).
Wilding, T. J. & Huettner, J. E. Differential antagonism of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-preferring and kainate-preferring receptors by 2,3-benzodiazepines. Mol. Pharmacol. 47, 582–587 (1995).
Bleakman, R. et al. Pharmacological discrimination of GluR5 and GluR6 kainate receptor subtypes by (3S,4aR,6R,8aR)-6-[2-(1(2)H-tetrazole-5-yl)ethyl]decahydroisodoquinoline-3 carboxylic-acid. Mol. Pharmacol. 49, 581–585 (1996).
Clarke, V. R. et al. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature 389, 599–603 (1997).
Bureau, I. et al. Kainate receptor-mediated responses in the CA1 field of wild-type and GluR6-deficient mice. J. Neurosci. 19, 653–663 (1999).
O'Neill, M. J. et al. Decahydroisoquinolines: novel competitive AMPA/kainate antagonists with neuroprotective effects in global cerebral ischemia. Neuropharmacology 37, 1211–1222 (1998).
Bolser, D. C. et al. The pharmacology of SCH 50911: a novel, orally-active GABAB receptor antagonist. J. Pharmacol. Exp. Ther. 274, 1393–1398 (1995).
Bortolotto, Z. A. et al. Kainate receptors are involved in synaptic plasticity. Nature 402, 297–301 (1999).
Lauridsen, J., Honoré, T. & Krogsgaard-Larsen, P. Ibotenic acid analogues. Synthesis, molecular flexibility, and in vitro activity of agonists and antagonists at central glutamic acid receptors. J. Med. Chem. 28, 668–672 (1985).
Stensbøl et al. Resolution, absolute stereochemistry and molecular pharmacology of the enantiomers of ATPA. Eur. J. Pharmacol. 380, 153–162 (1999).
Andersen, P., Sundberg, S. H., Sveen, O. & Wigström, H. Specific long-lasting potentiation of synaptic transmission in hippocampal slices. Nature 266, 736–737 (1997).
Aroniadou-Anderjaska, V., Post, R. M, Rogawski, M. A. & Li, H. Input-specific LTP and depotentiation in the basolateral amygdala. Neuroreport 12, 635–640 (2001).
Savander, V., Miettinen, R., LeDoux, J. E. & Pitkänen, A. Lateral nucleus of the rat amygdala is reciprocally connected with basal and accessory basal nuclei: a light and electron microscopic study. Neuroscience 77, 767–781 (1997).
Farb, C. R., Aoki, C. & LeDoux, J. E. Differential localization of NMDA and AMPA receptor subunits in the lateral and basal nuclei of the amygdala: a light and electron microscopic study. J. Comp. Neurol. 363, 86–108 (1995).
Fitzjohn, S. M. et al. Activation of group I mGluRs potentiates NMDA responses in rat hippocampal slices. Neurosci. Lett. 203, 211–213 (1996).
Hermans, E., Nahorski, S. R. & Challiss, R. A. Reversible and non-competitive antagonist profile of CPCCOEt at the human type 1α metabotropic glutamate receptor. Neuropharmacology 37, 1645–1647 (1998).
Weisskopf, M. G. & Nicoll, R. A. Presynaptic changes during mossy fibre LTP revealed by NMDA receptor-mediated synaptic responses. Nature 376, 256–259 (1995).
Sommer, B., Köhler, M., Sprengel, R. & Seeburg, P. H. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell 67, 11–19 (1991).
Bernard, A. et al. Q/R editing of the rat GluR5 and GluR6 kainate receptors in vivo and in vitro: evidence for independent developmental, pathological and cellular regulation. Eur. J. Neurosci. 11, 604–616 (1999).
Hume, R. I., Dingledine, R. & Heinemann, S. F. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science 253, 1028–1031 (1991).
Egebjerg, J. & Heinemann, S. F. Ca2+ permeability of unedited and edited versions of the kainate selective glutamate receptor GluR6. Proc. Natl. Acad. Sci. USA 90, 755–759 (1993).
Burnashev, N., Zhou, Z., Neher, E. & Sakmann, B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J. Physiol. (Lond.) 485 (Pt. 2), 403–418 (1995).
Bernard, A. & Khrestchatisky, M. Assessing the extent of RNA editing in the TMII regions of GluR5 and GluR6 kainate receptors during rat brain development. J. Neurochem. 62, 2057–2060 (1994).
Vignes, M. et al. The GluR5 subtype of kainate receptor regulates excitatory synaptic transmission in areas CA1 and CA3 of the rat hippocampus. Neuropharmacology 37, 1269–1277 (1998).
Contractor, A., Swanson, G. T., Sailer, A., O'Gorman, S. & Heinemann, S. F. Identification of the kainate receptor subunits underlying modulation of excitatory synaptic transmission in the CA3 region of the hippocampus. J. Neurosci. 20, 8269–8278 (2000).
Ouanounou, A. et al. Accumulation and extrusion of permeant Ca2+ chelators in attenuation of synaptic transmission at hippocampal CA1 neurons. Neuroscience 75, 99–109 (1996).
Bashir, Z. I., Alford, S., Davies, S. N., Randall, A. D. & Collingridge, G. L. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature 349, 156–158 (1991).
O'Connor, J. J., Rowan, M. J. & Anwyl, R. Long-lasting enhancement of NMDA receptor-mediated synaptic transmission by metabotropic glutamate receptor activation. Nature 367, 557–559 (1994).
Anwyl, R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res. Brain Res. Rev. 29, 83–120 (1999).
Lüscher, C., Nicoll, R. A., Malenka, R. C. & Muller, D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat. Neurosci. 3, 545–550 (2000).
Mainen, Z. F., Malinow, R. & Svoboda, K. Synaptic calcium transients in single spines indicate that NMDA receptors are not saturated. Nature 399, 151–155 (1999).
Ben-Ari, Y. & Cossart, R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 23, 580–587 (2000).
Frerking M. & Nicoll, R. A. Synaptic kainate receptors. Curr. Opin. Neurobiol. 10, 342–251 (2000).
Nicoll, R. A., Mellor, J., Frerking, M. & Schmitz, D. Neurobiology: kainate receptors and synaptic plasticity. Nature 406, 957 (2000).
Jones, K. A., Wilding, T. J., Huettner, J. E. & Costa, A. M. Desensitization of kainate receptors by kainate, glutamate and diasteriomers of 4-methylglutamate. Neuropharmacology 36, 853–863 (1997).
Chapman, P. F. & Chattarki, S. in The Amygdala. A Functional Analysis 2nd edn. (ed. Aggleton, J. P.) 117–153 (Oxford Univ. Press, Oxford, 2000).
Schuman, E. M. & Madison, D. V. Locally distributed synaptic potentiation in the hippocampus. Science 263, 532–536 (1994).
Engert, F. & Bonhoeffer, T. Synapse specificity of long-term potentiation breaks down at short distances. Nature 388, 279–284 (1997).
Scanziani, M., Malenka, R. C. & Nicoll, R. A. Role of intercellular interactions in heterosynaptic long-term depression. Nature 380, 446–450 (1996).
van der Kolk, B.A. The psychobiology of posttraumatic stress disorder. J. Clin. Psychiatry 58 (Suppl. 9), 16–24 (1997).
Tuunanen, J., Halonen, T. & Pitkänen, A. Status epilepticus causes selective regional damage and loss of GABAergic neurons in the rat amygdaloid complex. Eur. J. Neurosci. 8, 2711–2725 (1996).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates 4th edn. (Academic, San Diego, 1998).
Acknowledgements
H.L. and G.X. acknowledge the support of the Stanley Foundation Bipolar Network of the NAMI Research Institute. This work was supported in part by DAMD grant 17-00-1-0110 and USUHS RO88DG grant to H.L.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Chen, A., Xing, G. et al. Kainate receptor-mediated heterosynaptic facilitation in the amygdala. Nat Neurosci 4, 612–620 (2001). https://doi.org/10.1038/88432
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/88432
This article is cited by
-
Downregulation of kainate receptors regulating GABAergic transmission in amygdala after early life stress is associated with anxiety-like behavior in rodents
Translational Psychiatry (2021)
-
Molecular mechanisms of group I metabotropic glutamate receptor mediated LTP and LTD in basolateral amygdala in vitro
Psychopharmacology (2017)
-
Coactivation of thalamic and cortical pathways induces input timing–dependent plasticity in amygdala
Nature Neuroscience (2012)
-
Stress Impairs 5-HT2A Receptor-Mediated Serotonergic Facilitation of GABA Release in Juvenile Rat Basolateral Amygdala
Neuropsychopharmacology (2009)
-
Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania
Molecular Psychiatry (2008)