Abstract
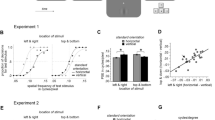
The visual system is thought to process luminance (first-order) and contrast (second-order) information by dedicated cortical streams. To explore the spatial characteristics of the second-order pathway, we examined the effect of adaptation on spatial localization in human subjects. We show that, unlike first-order adaptation, second-order positional adaptation via cortical mechanisms transfers across orientations but not across spatial frequencies. These results support physiological evidence that these two processing streams are distinct and suggest that the cortical mechanism mediating second-order positional adaptation maintains spatial frequency information but sums signals across orientations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hubel, D. H. & Wiesel, T. N. Receptive fields, binocular interaction, and functional architecture in the cat's visual cortex. J. Physiol. (Lond.) 160, 106–154 ( 1962).
Movshon, J. A., Thompson, I. A. & Tollhurst, D. J. Spatial summation in the receptive fields of simple cells in the cat's striate cortex. J. Physiol. (Lond.) 283, 53–77 (1978).
Spitzer, H. & Hochstein, S. Simple- and complex-cell response dependences on stimulation parameters. J. Neurophysiol. 53, 1244–1265 (1985).
Shapley, R. M. & Lennie, P. Spatial frequency analysis in the visual system. Annu. Rev. Neurosci. 8, 547 –583 (1985).
von der Heydt, R. & Peterhans, E. Mechanisms of contour perception in monkey visual cortex. I. Lines of pattern discontinuity. J. Neurosci. 9, 1731–1748 (1989).
von der Heydt, R. & Peterhans, E. & Dursteler, M. R. Periodic-pattern-selective cells in monkey visual cortex. J. Neurosci. 12, 1416–1434 (1992).
Merigan, W. H., Nealey, T.A & Maunsell, J. H. Visual effects of lesions of cortical area V2 in macaques. J. Neurosci. 13, 3180– 3191 (1993).
Shapley, R. M. Visual cortex: pushing the envelope. Nat. Neurosci. 1, 95–96 (1998).
Li-Ming, L. & Wilson, H. R. Fourier and non-Fourier pattern discrimination. Vision Res. 36, 1907– 1918 (1996).
Chubb, C. & Sperling, G. Drift-balanced random stimuli: A general basis for studying non-Fourier motion perception. J. Opt. Soc. Am. A 5, 1986–2007 (1988).
Turano, K. & Pantle, A. On the mechanism that encodes the movement of contrast variations: Velocity discrimination. Vision Res. 29, 207–221 ( 1974).
Derrington, A. M., Badcock, D. R. & Henning, G. B. Discriminating the direction of second-order motion at short stimulus durations. Vision Res. 33, 1785–1794 (1993).
Ledgeway, T. & Smith, A. T. Evidence for separate motion-detecting mechanisms for first- and second-order motion in human vision. Vision Res. 34, 2727–2740 (1994).
Vaina, L. M., Makris, N., Kennedy, D. & Cowey, A. The selective impairment of the perception of first-order motion by unilateral brain damage. Vis. Neurosci. 15, 333–348 (1998).
Zhou, Y.-X. & Baker, C. L. A processing stream in mammalian visual cortex neurons for non-Fourier responses. Science 261, 98–101 (1993).
Morgan, M. J. & Hotopf, W. H. Perceived diagonals in grids and lattices. Vision Res. 29, 1005– 1015 (1989).
Victor, J. D. & Conte, M. M. Spatial organization of nonlinear interactions in form perception. Vision Res. 31, 1457–1488 (1991).
Graham, N., Beck, J. & Sutter, A. Nonlinear processes in spatial-frequency channel models of perceived texture segregation: effects of sign and amount of contrast. Vision Res. 32, 719–743 (1992).
Levi, D. M. & Waugh, S. J. Position acuity with opposite-contrast polarity features: evidence for a nonlinear collector mechanism for position acuity. Vision Res. 36, 573– 588 (1996).
Olavarria, J. F., DeYoe, E. A., Knierim, J. J., Fox, J. M. & Van Essen, D. C. Neural responses to visual texture patterns in the middle temporal area of the macaque monkey. J. Neurophysiol. 68, 164–181 (1992).
Shapley, R. M. in Higher-Order Processing in the Visual System. Ciba Foundation Symposia 71–87 (Wiley, New York, 1994).
Mareschal, I. & Baker, C. L. A cortical locus for the processing of contrast-defined contours. Nat. Neurosci. 1, 150–154 (1998).
Pantle, A. & Sekuler, R. Size detecting mechanisms in human vision. Science 162, 1146– 1148 (1968).
Blakemore, C. & Campbell, F. W. On the existence of neurons in the human visual system selectively sensitive to the orientation and size of retinal images. J. Physiol. (Lond.) 203, 237–260 (1969).
Gibson, J. J. & Radner, M. Adaptation, aftereffect, and contrast in the perception of tilted lines. I. Quantitative studies. J. Exp. Psychol. 20, 453–467 (1937).
Moulden, B., Renshaw, J. & Mather, G. Two channels for flicker in the human visual system. Perception 13, 387–400 (1984).
McCollough, C. Color adaptation of edge detectors in the human visual system. Science 149, 1113–1114 ( 1965).
Pantle, A. Motion aftereffect magnitude as a measure of spatiotemporal response properties of direction-selective analysers. Vision Res. 14, 1229–1236 (1974).
Whitaker, D., McGraw, P. V. & Levi, D. M. The influence of adaptation on perceived visual location. Vision Res. 37, 2207–2216 (1997).
Sutherland, N. S. Figural after-effects and apparent size. J. Exp. Psychol. 13, 222–228 (1961).
Mareschal, I. & Baker, C. L. Temporal and spatial response to second-order stimuli in cat area 18. J. Neurophysiol. 80, 2811–2823 (1998).
Burton, G. J. Evidence for non-linear response process in the visual system from measurements on the thresholds of spatial beat frequencies. Vision Res. 13, 1211–1255 (1973).
Henning, G. B., Hertz, G. B. & Broadbent, D. E. Some experiments bearing on the hypothesis that the visual system analyses patterns in independent bands of spatial frequency. Vision Res. 15, 887–897 (1975).
Nachmias, J. N. & Rogowitz, B. E. Masking by spatially modulated gratings. Vision Res. 23, 1621–1629 (1983).
Derrington, A. M. & Badcock, D. R. Detecting spatial beats: non-linearity or contrast increment detection? Vision Res. 27, 343–348 ( 1986).
Klein, S. A. & Levi, D. M. Hyperacuity thresholds of 1 sec: Theoretical predictions and empirical validation. J. Opt. Soc. Am. A 2, 1170–1190 ( 1985).
Wilson, H. R. Responses of spatial mechanisms can explain hyperacuity. Vision Res. 26, 453–469 ( 1986).
Georgeson, M. A. Human vision combines oriented filters to compute edges. Proc. R. Soc. Lond. B 249, 235–245 (1992).
Thomas, J. P. & Olzak, L. A. Uncertainty experiments support the roles of second-order mechanisms in spatial frequency and orientation discriminations. J. Opt. Soc. Am. A 13, 689–696 (1996).
Nothdurft, H. C. in Structural and Functional Organization of the Neocortex (eds. Albowitz, B., Albus, K., Kuhnt, U., Nothdurft, H. C. & Wahle, P.) 375–384 (Springer, Berlin, 1994).
Sagi, D. in Early Vision and Beyond (eds. Papathomas, T. V., Chubb, C., Gorea, A. & Kowler, E.) 69–78 (MIT Press, Cambridge, Massachusetts, 1995).
Acknowledgements
P.V.M. is supported by a Vision Research Training Fellowship from the Wellcome Trust. D.M.L. is supported by grant RO1EY01728 from the National Eye Institute, NIH, Bethesda, Maryland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McGraw, P., Levi, D. & Whitaker, D. Spatial characteristics of the second-order visual pathway revealed by positional adaptation. Nat Neurosci 2, 479–484 (1999). https://doi.org/10.1038/8150
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/8150