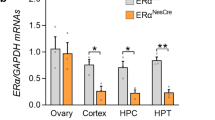
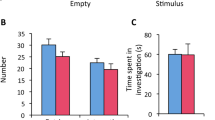
Abstract
The two genes coding for thyroid hormone receptors (TR) α 1 and β have opposite effects on female sex behaviors. Deletion of TRα 1 reduced them, whereas deletion of TRβ actually increased them. These results could not be attributed to altered levels of hormones in the blood, general alterations in estrogen responsiveness or altered general activity. Instead, they indicate a previously unknown molecular mechanism upon which the two TR genes exert opposite influences.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pfaff, D.W. Drive: Neural and Molecular Mechanisms for Sexual Motivation (MIT Press, Cambridge, Massachusetts, 1999).
Zhu, Y., Yen, P., Chin, W. & Pfaff, D. Estrogen and thyroid hormone interaction on regulation of gene expression. Proc. Natl. Acad. Sci. USA 93, 12587–12592 (1996).
Dellovade, T., Kia, H., Zhu, Y.-S. & Pfaff, D. Thyroid hormone coadministration inhibits the estrogen-stimulated elevation of preproenkephalin mRNA in female rat hypothalamic neurons. Neuroendocrinology 70, 168–174 (1999).
Dellovade, T., Zhu, Y. & Pfaff, D. Thyroid hormones and estrogen affect oxytocin gene expression in hypothalamic neurons. J. Neuroendocrinol. 11, 1–10 (1999).
Dellovade, T., Zhu, Y., Krey, L. & Pfaff, D. Thyroid hormone and estrogen interact to regulate behavior. Proc. Natl. Acad. Sci. USA 93, 12581–12586 (1996).
Morgan, M. & Pfaff, D. Thyroid hormones reduce lordosis behavior in female mice. Horm. Behav. (in press).
Dahl, G., Evans, N., Thrun, L. & Karsch, F. Thyroxine is permissive to seasonal transitions in reproductive neuroendocrine activity in the ewe. Biol. Reprod. 52, 690–696 (1995).
Webster, J., Moenter, S., Barrell, G., Lehman, M. & Karsch, J. Role of the thyroid gland in seasonal reproduction. III. Thyroidectomy blocks seasonal suppression of gonadotropin-releasing hormone secretion in sheep. Endocrinology 129, 1635–1643 (1991).
Moenter, S., Woodfill, C. & Karsch, F. Role of the thyroid gland in seasonal reproduction: Thyroidectomy blocks seasonal suppression of reproductive neuroendocrine activity in ewes. Endocrinology 128, 1337–1344 (1991).
Thrun, L., Dahl, G., Evans, N. & Karsch, F. Acritical period for thyroid hormone action on seasonal changes in reproductive neuroendocrine function in the ewe. Endocrinology 138, 3402–3409 (1997).
Webster, J., Moenter, S., Woodfill, C. & Karsch, J. Role of the thyroid gland in seasonal reproduction. II. Thyroxine allows a season-specific suppression of gonadotropin secretion in sheep. Endocrinology 129, 176–183 (1991).
Goldsmith, A. & Nichols, T. Thyroidectomy prevents the development of photorefractoriness and the associated rise in plasma prolactin in starlings. Gen. Comp. Endocrinol. 54, 256–263 (1984).
Goldsmith, A. & Nichols, T. Thyroxine induces photorefractoriness and stimulates prolactin secretion in European starlings (Sturnus vulgaris) J. Endocrinol. 101, 1–3 (1984).
Johansson, C., Vennström, B. & Thorén, P. Evidence that decreased heart rate in thyroid hormone receptor α1–deficient mice is an intrinsic defect. Am. J. Physiol. 275, R640–646 (1998).
Johansson, C., Gothe, S., Forrest, D., Vennström, B. & Thorén, P. Cardiovascular phenotype and temperature control in mice lacking thyroid hormone receptor-beta or both alpha1 and beta. Am. J. Physiol. 276, H2006–2012 (1999).
Forrest, D. et al. Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor β: evidence for tissue-specific modulation of receptor function. EMBO J. 15, 3006–3015 (1996).
Wikstrom, L. et al. Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor EMBO J. 17, 455–461 (1998).
Rusch, A., Erway, L., Oliver, D., Vennstrom, B. & Forrest, D. Thyroid hormone receptordependent expression of a potassium conductance in inner hair cells at the onset of hearing. Proc. Natl. Acad. Sci. USA 95, 15758–15762 (1998).
Sandhofer, C., Schwartz, H., Mariash, C., Forrest, D. & Oppenheimer, J. Beta receptor isoforms are not essential for thyroid hormone-dependent acceleration of PCP-2 and myelin basic protein gene expression in the developing brains of neonatal mice. Mol. Cell. Endocrinol. 137, 109–115 (1998).
Pedersen, C., Jirikowski, G., Caldwell, J. & Insel, T. Oxytocin in maternal, sexual and social behavior. Ann. NY Acad. Sci. 652 (1992).
Carter, C.S., Lederhendler, I. & Kirkpatrick, B. The integrative neurobiology of affiliation. Introduction Ann. NY Acad. Sci. 807, xiii–xviii (1997).
Young, L., Wang, Z. & Insel, T. Neuroendocrine bases of monogamy. Trends Neurosci. 21, 71–75 (1998).
Fahrbach, S., Morrell, J. & Pfaff, D. Effect of varying the duration of pre-test cage habituation on oxytocin induction of short-latency maternal behavior. Physiol. Behav. 37, 135–139 (1986).
Neumann, I.D. et al. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J. Physiol. (Lond.) 508, 289–300 (1998).
Neumann, I., Douglas, A., Pittman, Q., Russell, J. & Landgraf, R. Oxytocin released within the supraoptic nucleus of the rat brain by positive feedback action is involved in parturition-related events. J. Neuroendocrinol. 8, 227–233 (1996).
Gauthier, K. et al. Different functions for the thyroid hormone receptors TRalpha and TRbeta in the control of thyroid hormone production and post-natal development. EMBO J. 18, 623–631 (1999).
Göthe, S. et al. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth and bone maturation. Genes Dev. 13, 1329–1341 (1999).
Mangelsdorf, D. et al. . Overview: the nuclear receptor superfamily: the second decade. Cell 83, 835–840 (1995).
Horlein, A. et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 377, 397–404 (1995).
Cohen, R., Wondisford, F. & Hollenberg, A. Two separate NCoR (Nuclear Receptor Corepressor) interaction domains mediate corepressor action on thyroid hormone response elements. Mol. Endocrinol. 12, 1567–1581 (1998).
Barros, A. C. et al. Absence of thyroid hormone receptor beta – retinoid X receptor interactions in auditory function and in the pituitary–thyroid axis. Neuroreport 9, 2933–2937 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dellovade, T., Chan, J., Vennstrom, B. et al. The two thyroid hormone receptor genes have opposite effects on estrogen-stimulated sex behaviors. Nat Neurosci 3, 472–475 (2000). https://doi.org/10.1038/74846
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/74846
This article is cited by
-
Thyroid hormone: sex-dependent role in nervous system regulation and disease
Biology of Sex Differences (2021)
-
Neonatal hyperthyroidism on rat heart: interrelation with nitric oxide and sex
Journal of Endocrinological Investigation (2015)
-
Thyroid hormones modulate the hypothalamo–hypophyseal–gonadal axis in teleosts: Molecular insights
Fish Physiology and Biochemistry (2007)
-
Lack of thyroid hormone receptor α1 is associated with selective alterations in behavior and hippocampal circuits
Molecular Psychiatry (2003)
-
Estrogen-like effects of thyroid hormone on the regulation of tumor suppressor proteins, p53 and retinoblastoma, in breast cancer cells
Oncogene (2002)