Abstract
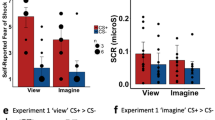
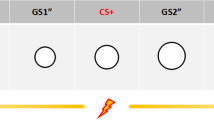
We examined the neural substrates involved when subjects encountered an event linked verbally, but not experientially, to an aversive outcome. This instructed fear task models a primary way humans learn about the emotional nature of events. Subjects were told that one stimulus (threat) represents an aversive event (a shock may be given), whereas another (safe) represents safety (no shock will be given). Using functional magnetic resonance imaging (fMRI), activation of the left amygdala was observed in response to threat versus safe conditions, which correlated with the expression of the fear response as measured by skin conductance. Additional activation observed in the insular cortex is proposed to be involved in conveying a cortical representation of fear to the amygdala. These results suggest that the neural substrates that support conditioned fear across species have a similar but somewhat different role in more abstract representations of fear in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Davis, M. Neurobiology of fear responses: the role of the amygdala. J. Neuropsychiatry Clin. Neurosci. 9, 382–402 (1997).
Kapp, B. S., Pascoe, J. P. & Bixler, M. A. in The Neuropsychology of Memory (eds. Butters, N. & Squire, L. S.) 473–488 (Guilford, New York, 1984).
LeDoux, J. E. The Emotional Brain (Simon & Schuster, New York, 1996)
Bechara, A. et al. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science 269, 1115–1118 (1995)
LaBar, K. S., LeDoux, J. E., Spencer, D. D. & Phelps, E. A. Impared fear conditioning following unilateral temporal lobectomy in humans. J. Neurosci. 15, 6846–6855 (1995).
Deane, G. E. Human heart rate responses during experimentally induced anxiety. J. Exp. Psychol. 61, 489–493 (1961).
Grillon, C., Ameli, R., Woods, S. W., Merikangas, K. & Davis, M. Fear potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology 28, 588–595 (1991).
Grillon, C. & Davis, M. Effects of stress and shock anticipation on prepulse inhibition of the startle reflex. Psychophysiology 34, 511–517 (1997).
Breiter, H. C. et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron 17, 875–887 (1996).
LaBar, K. S. Gatenby, J. C., Gore, J. C., LeDoux, J. E. & Phelps, E. A. Human amygdala activation during conditioned fear acquisition and extinction: a mixed trial fMRI study. Neuron 20, 937–945 (1998).
Buchel, C., Morris, J., Dolan, R. J. & Friston, K. J. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron 20, 947–957 (1998).
Quirk G. J., Armony, J. L. & LeDoux, J. E. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron 19, 613–624 (1997).
Pascoe, J. P. & Kapp, B. S. Electrophysiological characteristics of amygdaloid central nucleus neurons in the awake rabbit. Brain Res. Bull. 14, 331–338 (1985).
Maeda, H. Morimoto, H. & Yanagimoto, K. Response characteristics of amygdaloid neurons provoked by emotionally significant environmental stimuli in cats, with special reference to response durations. Can. J. Physiol. Pharmacol. 71, 374–378 (1993).
Collins, D. R. & Pare, D. Reciprocal changes in the firing probability of lateral and central medial amygdala neurons. J. Neurosci. 19, 836–844 (1999).
Rauch, S. L., van der Kolk, B. A., Fisler, R. E. & Alpert, N. M. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch. Gen. Psychiatry 53, 380–387 (1996).
Morris, J. S., Ohmann, A. & Dolan, R. J. Conscious and unconscious emotional learning in the human amygdala. Nature 393, 467–470 (1998).
Angrilli, A. et al. Startle reflex and emotion modulation impairment after a right amygdala lesion. Brain 119, 1991–2000 (1997).
Funayama, E. S., Grillon, C. G., Davis, M. & Phelps, E. A. A double dissociation in the affective modulation of startle in humans: effects of unilateral temporal lobectomy. J. Cogn. Neurosci. (in press).
Shi, C. J. & Cassell, M. D. Cascade projections from somatosensory cortex to the rat basolateral amygdala via the parietal insular cortex. J. Comp. Neurol. 399, 469–491 (1998).
Ploghaus, A. et al. Dissociating pain from its anticipation in the human brain. Science 284, 1979–1981 (1999).
Romanski, L. M. & LeDoux, J. E. Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. J. Neurosci. 12, 4501–4509 (1992).
Shi, C.-J. & Davis, M. Pain pathways involved in fear conditioning measured with fear-potentiated startle: lesion studies. J. Neurosci. 19, 420–430 (1999).
Bush, G. et al. The counting Stroop: an interference task specialized for functional neuroimaging: validation study with functional MRI. Hum. Brain Mapp. 6, 270–282 (1998)
Lane, R. D., Fink G. R., Gereon, R., Chau, P. M. & Dolan, R. J. Neural activation during selective attention to subjective emotional responses. Neuroreport 8, 3969–3972 (1997).
Talairach, J. & Tournoux, P. Co-Planar Stereotaxic Atlas of the Human Brain (Thieme, New York, 1988).
Bullmore, E. et al. Statistical methods of estimation and interference for functional MR image analysis. Magn. Reson. Med. 35, 261–277 (1996).
Constable, R. T. et al. Quantifying and comparing region-of-interest activation patterns in functional brain imaging: methodology considerations. Mag. Reson. Imaging 16, 289–300 (1998).
Acknowledgements
The authors acknowledge the inspiration of Charles Oakley. We also thank M. Nordan and K. LaBar for work on a pilot study. This research was supported by McDonnell-Pew Program in Cognitive Neuroscience 97-26 and National Institutes of Health grants MH50812 to E.A.P. and NS33332 to J.C.G.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Phelps, E., O'Connor, K., Gatenby, J. et al. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci 4, 437–441 (2001). https://doi.org/10.1038/86110
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/86110
This article is cited by
-
Use of Machine Learning Methods to Analyze Patterns of Brain Activity during Assessment of the Self and Others
Neuroscience and Behavioral Physiology (2023)
-
Embodying the avatar of an omnipotent agent modulates the perception of one’s own abilities and enhances feelings of invulnerability
Scientific Reports (2022)
-
The influence of EEG oscillations, heart rate variability changes, and personality on self-pain and empathy for pain under placebo analgesia
Scientific Reports (2022)
-
Insular cortex corticotropin-releasing factor integrates stress signaling with social affective behavior
Neuropsychopharmacology (2022)
-
Prefrontal cortex, amygdala, and threat processing: implications for PTSD
Neuropsychopharmacology (2022)