Abstract
We produced CA1-specific NMDA receptor 1 subunit-knockout (CA1-KO) mice to determine the NMDA receptor dependence of nonspatial memory formation and of experience-induced structural plasticity in the CA1 region. CA1-KO mice were profoundly impaired in object recognition, olfactory discrimination and contextual fear memories. Surprisingly, these deficits could be rescued by enriching experience. Using stereological electron microscopy, we found that enrichment induced an increase of the synapse density in the CA1 region in knockouts as well as control littermates. Therefore, our data indicate that CA1 NMDA receptor activity is critical in hippocampus-dependent nonspatial memory, but is not essential for experience-induced synaptic structural changes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Rosene, D. L. & Van Hoesen, G. W. in Cerebral Cortex Vol. 6: Further Aspects of Cortical Function, Including Hippocampus (eds. Jones, E. G. & Petes, A.) 345–447 (Plenum, New York, 1987).
Scoville, W. B. & Milner, B. Loss of recent memory after bilateral hippocampal lesion. J. Neurol. Neurosurg. Psychiatry 20, 11–12 ( 1957).
Zola-Morgan, S., Squire, L. R. & Amaral, D. G. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J. Neurosci. 6, 2950– 2967 (1986).
Squire L. R. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 99, 195– 231 (1992).
Mumby, D. G. et al. Ischemia-induced object-recognition deficits in rats are attenuated by hippocampal ablation before or soon after ischemia. Behav. Neurosci. 110, 266–281 ( 1996).
Wood, E. R., Dudchenko, P. A. & Eichenbaum, H. The global record of memory in hippocampal neuronal activity. Nature 397, 613– 616 (1999).
Moriyoshi, K. et al. Molecular cloning and characterization of the rat NMDA receptor . Nature 354, 31–37 (1991).
Nicoll, R. A. & Malenka, R. C. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann. NY Acad. Sci. 868, 515–525 ( 1999).
Bear, M. F. & Malenka, R. C. Synaptic plasticity: LTP and LTD. Curr. Opin. Neurobiol. 4, 389– 399 (1994).
Tang, Y.-P et al. Genetic enhancement of learning and memory in mice. Nature 401, 63–69 ( 1999).
Tsien, J. Z. et al. Subregion- and cell type-restricted gene knockout in mouse brain. Cell 87, 1317–1326 (1996).
Tsien, J. Z., Huerta, P. T. & Tonegawa, S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87, 1327–1338 (1996).
McHugh, T. J., Blum, K. I., Tsien, J. Z., Tonegawa, S. & Wilson, M. A. Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice. Cell 87, 1339–1349 (1996).
Woolf, N. J. A structural basis for memory storage in mammals. Prog. Neurobiol. 55, 59–77 ( 1998).
Bailey, C. H. & Kandel, E. R. Structural changes accompanying memory storage. Annu. Rev. Physiol. 55, 397–426 (1993).
Moser, M. B., Trommald, M. & Andersen, P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc. Natl. Acad. Sci. USA 91, 12673–12675 (1994).
Goodman, C. S. & Shatz, C. J. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell 72, Suppl. 77–98 (1993).
Li, Y., Erzurumlu, R. S., Chen, C., Jhaveri, S. & Tonegawa, S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice . Cell 76, 427–437 (1994).
Kirov, S. A. & Harris, K. M. Dendrites are more spiny on mature hippocampal neurons when synapses are inactivated. Nat. Neurosci. 10, 878–883 ( 1999).
Sorra, K. E. & Harris, K. M. Stability in synapse number and size at 2 hr after long-term potentiation in hippocampal area CA1. J. Neurosci. 18, 658–671 (1998).
Mansuy, I. M., Mayford, M., Jacob, B., Kandel, E. R. & Bach, M. E. Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell 92, 39–49 ( 1998).
Kogan, J. H. et al. Spaced training induces normal long-term memory in CREB mutant mice. Curr. Biol. 7, 1– 11 (1996).
Strupp, B. J. & Levitsky, D. A. Social transmission of food preferences in adult hooded rats (Rattus norvegicus). J. Comp. Psychol. 98, 257–266 (1984).
Bunsey, M. & Eichenbaum, H. Selective damage to the hippocampal region blocks long-term retention of a natural and nonspatial stimulus-stimulus association. Hippocampus 5, 546– 556 (1995).
Phillips, R. G. & LeDoux, J. E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning . Behav. Neurosci. 106, 274– 285 (1992).
Kempermann, G., Kuhn, G. H. & Gage, F. H. More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493– 495 (1997).
Dalrymple-Alford, J. C. & Benton, D. Preoperative differential housing and dorsal hippocampal lesions in rats. Behav. Neurosci. 98, 23–34 ( 1984).
Rosenzweig, M. R. Environmental complexity, cerebral change, and behavior. Am. Psychol. 21, 321–332 ( 1966).
Diamond, M. C. Enriching Heredity: The Impact of the Environment on the Anatomy of the Brain (Free Press, New York, 1988).
Fiala, B. A., Joyce, J. N. & Greenough, W. T. Environmental complexity modulates growth of granule cell dendrites in developing but not adult hippocampus of rats. Exp. Neurol. 59, 372–383 (1978).
Greenough, W. T., Withers, G. S. & Wallace, C. S. in The Biology of Memory (eds. Squire, L. R. & Lindenbaum, E.) 159–185 (Schattauer, Stuttgart, 1990).
Gabbott, P. L & Somogyi, J. The “single” Golgi impregnation procedure: methodological description. J. Neurosci. Methods 11, 221–230 (1984).
Sorra, K. E., Fiala, J. C. & Harris, K. M. Critical assessment of the involvement of perforations, spinules, and spine branching in hippocampal synapse formation. J. Comp. Neurol. 398, 225–240 (1998).
Sterio, D. C. The unbiased estimation of number and sizes of particles using the disector . J. Microsc. 134, 127– 136 (1984).
DeGroot, D. M. G. & Bierman, E. P. B. A critical evaluation of methods for estimating the numerical density of synapses. J. Neurosci. Methods 18, 79–101 (1986).
Gray, E. G. Axosomatic and axodendritic synapses of the cerebral cortex: An electron microscopic study. J. Anat. 83, 420– 433 (1959).
Nicoll, R. A. & Malenka, R. C. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature 377, 115–118 (1995).
Stevens, C. F. & Sullivan, J. Synaptic plasticity . Curr. Biol. 8, R151–153 (1998).
Woolley, C. S., Gould, E., Frankfurt, M. & McEwen, B. S. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons . J. Neurosci. 10, 4035– 4039 (1990).
Franklin, K. B. J. & Paxinos, G. The mouse brain in stereotaxic coordinates. (Academic, San Diego, 1997 ).
Gundersen, H. J. G. Notes on the estimation of the numerical density of arbitrary profiles: the edge effect. J. Microsc. 111, 219– 223 (1977).
Acknowledgements
We thank E. Gould for help with stereology and statistics and reading the manuscript, and P. Tanapat for technical advice concerning the Golgi method. This work was supported in part by a postdoctoral fellowship from Fondation pour la Recherche Medicale to C.R. and by grants from Princeton University, Beckman Foundation and NIH to J.Z.T.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Detailed materials and methods
Animals. The CA1-KO and normal control mice were produced as described1. Because electrophysiological, behavioral and anatomical experiments showed similar results for wild-type and Cre transgenic mice, these two genotypes of mice were pooled together as normal controls. The animals used for the behavioral tests were littermates of similar age (control mice, 3-5.5 months; CA1-KO mice, 3-4.5 months) and were maintained under standard conditions (23 ± 1°C, 50 ± 5% humidity) with a light-dark cycle of 12:12 h. All behavioral experiments except tests of social transmission of food preference were conducted in a soundproof room. Additionally, a black curtain was used to occlude possible environmental visual cues and experimenters from the mice's view during the tests. Throughout the experiment, experimenters were blind to the genotype of each animal.
Enriched environment training. Adult littermates were randomly distributed into two experimental groups assigned to one of the two experimental sets. One group was kept in standard cages (naive group) and the other group trained in an enriched environment for three hours daily for two months (enriched group). The enrichment training was carried out in specially designed boxes (1.5 m x 0.8 m x 0.8 m), in which various toys, running wheels and small houses were changed every other day to encourage exploration. Additionally, all animals received food and water ad libidum at various locations within the box.
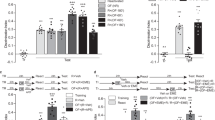
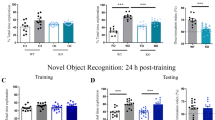
Novel-object recognition task. After the habituation, each animal was allowed 15 min to explore the box, into which 2 objects had been placed symmetrically. The animal was returned to its home cage immediately after training, and was subsequently subjected to periodic retention tests. In a retention test, one of the familiar objects was replaced by a novel object, and the times spent exploring each of the objects throughout a five-min period were recorded. Exploratory preference was calculated as a ratio in percentage of the time spent exploring any object (training) or the novel one (retention) over 50% of the total time spent exploring both objects. ANOVA and post-hoc Dunnett's test were used to determine genotype effects on the behavioral responses.
Social transmission of food preference. In this experiment, wild-type 'demonstrator' mice interacted with 'observer' (control or CA1-KO) mice subsequently tested for memory according to a described procedure2. Observers and demonstrators were selected from different cages. For at least three days before the test, observer and demonstrator animals were trained to eat from a food cup on the ground of their home cages. After ~24-h food deprivation in their home cages, half of the demonstrators were given cinnamon-scented food (1% per weight) and the other half were fed with cocoa-scented food (2% per weight) for 2 h. Remaining food was weighed; only mice that ate more than 0.5 g of the scented food were then used as demonstrators in the formal experiments. Each pair of one demonstrator mouse and one observer mouse was then allowed to interact in another cage for two periods (five min each) separated by one h. To avoid a bias for one scent, half of the observer animals in each genotype interacted with cinnamon-fed demonstrators and the other half with cocoa-fed demonstrators. After interaction, the observers were deprived of food for ~24 h and then subjected to memory testing. During testing, observers were offered both cinnamon- and cocoa-scented food from cups placed at either end of the cage for 2 h, and consumption of each food was subsequently measured. Preference was calculated as percentage ratio of the amount of preferred food consumed over 50% of the total amount of food consumed. Typically, the preferred food had the same scent as food eaten by the demonstrator with whom the observer had interacted. ANOVA was used to determine genotype effects on the behavioral responses.
Fear-conditioning task. The system used in this study consisted of a shock chamber, a multi-tone generator with a speaker, an electrical-shock producer, a photobeam scanner, and a workstation loaded with TruScan 98 software. Behavioral responses observed by experimenters were also automatically recorded by the computerized scanner system. After habituation for one day, the animals were subjected to conditioned stimulus/unconditioned stimulus pairing in the chamber. Retention tests for both contextual and cued conditioning were carried out 24 h after training. Freezing responses, judged as complete immobilizations of the body (except for respiratory movements) were sampled every five s and were recorded simultaneously by both the experimenters and the photobeam-scanner system. ANOVA and post-hoc Dunnett's test were used to determine the effect of genotype on behavioral responses.
Histology. For electronic microscopy experiments, control groups were constituted of males, 3.5-5.5 months old. The CA1-KO naive mice were 3 males (4.5, 4 and 4 months) and 1 female (3.5 months), and the CA1-KO enriched mice were 3 males (5, 4.5 and 3.5 months) and 1 female (4 months). Because CA1 dendritic spine density in rats fluctuates over the estrous cycle3, females were killed during proestrus, the stage at which spine numbers of female rats approach those of male rats (although it is not known whether the same phenomenon exists in mice). Animals were anesthetized and perfused with 2% paraformaldehyde, 2% glutaraldehyde and 1.5% saturated picric acid in 0.1 M phosphate buffer (pH 7.4) and postfixed overnight in the same solution. Following a modified version of the single-section Golgi impregnation technique4, 100 μm-thick coronal sections were cut with a vibratome into a bath of 3% potassium dichromate in distilled water and incubated overnight in this solution. The sections were then rinsed in distilled water and mounted on ungelatinized glass slides. The slide assemblies were incubated in the dark in a solution of 1.5% silver nitrate for 2 days. A coverslip was placed over the sections and glued at the four corners of the slide. These slide assemblies were incubated in the dark in a solution of 1.5% silver nitrate over 2 nights. Coverslips were then removed, and the sections were rinsed, dehydrated and re-coverslipped. For Golgi experiments, only animals showing staining that met the criteria of an excellent impregnation (see Data analysis: spine density) were used; others were excluded from subsequent analysis. As a result, three animals per group were examined.
For electron microscopy, we used a vibratome to cut 250 μm-thick coronal sections into a bath of 0.1 M sodium cacodylate buffer. Hippocampal sections corresponding to figures 47-50 of the mouse brain atlas5 were dissected, rinsed in distilled water and postfixed in 1% osmium tetroxide followed with 1% ferrocyanide-reduced osmium tetroxide. Tissues were rinsed, stained en bloc overnight in 1% aqueous uranyl acetate, rinsed and dehydrated and subsequently infiltrated and flat embedded with Embed 812 resin. Sections cut at 0.5 μm and stained with 1% toluidine blue were examined to determine how the block should be trimmed for ultrathin sectioning so as to include the apex of the CA1 pyramidal cell layer, from the stratum radiatum to the stratum lacunosum moleculare. Ultrathin sections were cut with a diamond knife, placed on carbon Formvar-coated slot grids and stained with 1% uranyl acetate and lead citrate. The sections were examined with a JEOL 100C electron microscope and micrographs were taken at 10,000X magnification. Every effort was made to obtain uniform section thickness at the time of cutting; however, thickness was estimated post hoc from scanning EM measurements to correct for potential bias. Serial sections (9-11 sections per block) from different blocks were placed on 400-mesh copper grids and sputter coated with gold and palladium. They were then examined with a scanning electron microscope at a tilt angle of 40°, providing a side view of the sections at 85°. Images were taken at 20,000X and the thickness was measured at various positions along the sections. Average estimated section thickness ranged from 0.087 to 0.105 μm depending on the series.
Data analysis: spine density. Golgi-impregnated dendritic segments selected for analysis were located 100-250 μm from the pyramidal cell bodies in the stratum radiatum, belonged to a thoroughly impregnated pyramidal neuron, remained approximately in the plane of focus and were >10 μm in length and 0.80-1 μm in width. These selection criteria were applied in an effort to restrict analyses to similar populations of neurons in each animal. The CA1 pyramidal cells were identified by their location and their spiny apical and basilar dendrites. Appropriate apical segments were photographed at 1,000X and the number of dendritic spines visible along each segment was counted. The length of each dendritic segment was measured from the pictures with Image-Pro software, and data were expressed as number of spines per ten μm of dendrite. Calculations of spine density were obtained by measuring dendritic segments matching the above criteria (control enriched n = 37, CA1-KO naive n = 38, CA1-KO enriched n = 41 and normal control naive n = 43) from age- and sex-balanced sets of 3 animals from each group. These segments represented a total dendritic length of 1001-1284 μm per group of mice.
Data analysis: synapse density. Synapse density was estimated by the disector method6,7. From each brain, 15 electron micrographs of separate regions within the stratum radiatum, 100-250 μm from the pyramidal cell layer, were taken from different sections at 10,000X on a JEOL 100C electron microscope to create 15 'reference' planes. Regions containing cell bodies or blood vessels were intentionally avoided. To prevent possible synapse-density gradients within the apical dendritic zone from complicating our data, we systematically balanced sampled images for apicobasal location within the stratum radiatum. The micrographs covered an area of approximately 2100 μm2 per animal. Micrographs of exactly the same regions were taken on an adjacent section to create 'look-up' planes. The number of synapses contained in a 'reference' plane but absent in the corresponding 'look-up' plane (Qi) was counted to determine the number of synapses present within the volume defined by the 'reference' plane, the 'look-up' plane and the distance between them (hi). Two sides of the rectangular picture were assigned randomly as inclusion or exclusion edges to create a counting frame that minimized potential edge effects across samples8. To increase the sampling reliability, 606-1043 synapses were counted over a total area of 8,400-14,700 μm2 from 600 electron micrographs, corresponding to 606-1043 synapses for each experimental group of mice. Section thickness, (hi) was determined by scanning electron microscope measurements (see Histology). Estimated synapse density (est Nv) was calculated as:
est NV = ∑Qi/∑(Aixhi).
REFERENCES in Supplementary Information
-
1.
Tsien, J. Z. et al. Subregion- and cell type-restricted gene knockout in mouse brain. Cell 87, 1317-1326 (1996).
-
2.
Kogan, J. H. et al. Spaced training induces normal long-term memory in CREB mutant mice. Curr. Biol. 7, 1-11 (1996).
-
3.
Woolley, C. S., Gould, E., Frankfurt, M. & McEwen, B. S. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci. 10, 4035-4039 (1990).
-
4.
Gabbott, P. L & Somogyi, J. The "single" Golgi impregnation procedure: methodological description. J. Neurosci. Methods 11, 221-230 (1984).
-
5.
Franklin, K. B. J. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates (Academic, San Diego, 1997).
-
6.
Sterio, D. C. The unbiased estimation of number and sizes of particles using the disector. J. Microsc. 134, 127-136 (1984).
-
7.
DeGroot, D. M. G. & Bierman, E. P. B. A critical evaluation of methods for estimating the numerical density of synapses. J. Neurosci. Methods 18, 79-101 (1986).
-
8.
Gundersen, H. J. G. Notes on the estimation of the numerical density of arbitrary profiles: the edge effect. J. Microsc. 111, 219-223 (1977).
Rights and permissions
About this article
Cite this article
Rampon, C., Tang, YP., Goodhouse, J. et al. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci 3, 238–244 (2000). https://doi.org/10.1038/72945
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/72945
This article is cited by
-
Acute Administration of HIV-1 Tat Protein Drives Glutamatergic Alterations in a Rodent Model of HIV-Associated Neurocognitive Disorders
Molecular Neurobiology (2024)
-
Environmental Enrichment in Stroke Research: an Update
Translational Stroke Research (2024)
-
Dynamic and stable hippocampal representations of social identity and reward expectation support associative social memory in male mice
Nature Communications (2023)
-
Excitation–transcription coupling, neuronal gene expression and synaptic plasticity
Nature Reviews Neuroscience (2023)
-
When deciding to cooperate by direct reciprocity, Norway rats sometimes benefit from olfactory competence and seem not impaired by insufficient cognitive abilities
Animal Cognition (2023)