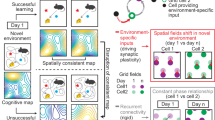
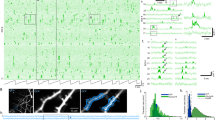
Abstract
Synaptic integrative mechanisms have profound effects on electrical signaling in the brain that, although largely hidden from recording methods that observe the spiking activity of neurons, may be critical for the encoding, storage and retrieval of information. Here we review roles for synaptic integrative mechanisms in the selection, generation and plasticity of place and grid fields, and in related temporal codes for the representation of space. We outline outstanding questions and challenges in the testing of hypothesized models for spatial computation and memory.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
O'Keefe, J. & Nadel, L. The Hippocampus as a Cognitive Map (Clarendon Press, 1978).
Redish, A.D. Beyond the Cognitive Map: From Place Cells to Episodic Memory (MIT Press, 1999).
Spruston, N. Pyramidal neurons: dendritic structure and synaptic integration. Nat. Rev. Neurosci. 9, 206–221 (2008).
Häusser, M., Spruston, N. & Stuart, G.J. Diversity and dynamics of dendritic signaling. Science 290, 739–744 (2000).
Chadderton, P., Schaefer, A.T., Williams, S.R. & Margrie, T.W. Sensory-evoked synaptic integration in cerebellar and cerebral cortical neurons. Nat. Rev. Neurosci. 15, 71–83 (2014).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521, 436–444 (2015).
Häusser, M. & Mel, B. Dendrites: bug or feature? Curr. Opin. Neurobiol. 13, 372–383 (2003).
O'Keefe, J., Burgess, N., Donnett, J.G., Jeffery, K.J. & Maguire, E.A. Place cells, navigational accuracy, and the human hippocampus. Phil. Trans. R. Soc. Lond. B 353, 1333–1340 (1998).
Hafting, T., Fyhn, M., Molden, S., Moser, M.-B. & Moser, E.I. Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806 (2005).
Harvey, C.D., Collman, F., Dombeck, D.A. & Tank, D.W. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461, 941–946 (2009).By developing methods for patch-clamp recording from CA1 pyramidal cells in awake mice navigating in a virtual environment, this study revealed the membrane potential dynamics that underlie place cell firing.
Schmidt-Hieber, C. & Häusser, M. Cellular mechanisms of spatial navigation in the medial entorhinal cortex. Nat. Neurosci. 16, 325–331 (2013).
Domnisoru, C., Kinkhabwala, A.A. & Tank, D.W. Membrane potential dynamics of grid cells. Nature 495, 199–204 (2013).
Hartley, T., Burgess, N., Lever, C., Cacucci, F. & O'Keefe, J. Modeling place fields in terms of the cortical inputs to the hippocampus. Hippocampus 10, 369–379 (2000).
Burgess, N. & O'Keefe, J. Models of place and grid cell firing and theta rhythmicity. Curr. Opin. Neurobiol. 21, 734–744 (2011).
Solstad, T., Moser, E.I. & Einevoll, G.T. From grid cells to place cells: a mathematical model. Hippocampus 16, 1026–1031 (2006).
Eichenbaum, H., Dudchenko, P., Wood, E., Shapiro, M. & Tanila, H. The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron 23, 209–226 (1999).
Burgess, N., Maguire, E.A. & O'Keefe, J. The human hippocampus and spatial and episodic memory. Neuron 35, 625–641 (2002).
Chrobak, J.J., Lörincz, A. & Buzsáki, G. Physiological patterns in the hippocampo-entorhinal cortex system. Hippocampus 10, 457–465 (2000).
O'Keefe, J. Place units in the hippocampus of the freely moving rat. Exp. Neurol. 51, 78–109 (1976).
Skaggs, W.E., McNaughton, B.L., Wilson, M.A. & Barnes, C.A. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6, 149–172 (1996).
Chadwick, A., van Rossum, M.C. & Nolan, M.F. Flexible theta sequence compression mediated via phase precessing interneurons. eLife 5, e20349 (2016).
Ziv, Y. et al. Long-term dynamics of CA1 hippocampal place codes. Nat. Neurosci. 16, 264–266 (2013).
Rich, P.D., Liaw, H.-P. & Lee, A.K. Place cells. Large environments reveal the statistical structure governing hippocampal representations. Science 345, 814–817 (2014).
Thompson, L.T. & Best, P.J. Place cells and silent cells in the hippocampus of freely-behaving rats. J. Neurosci. 9, 2382–2390 (1989).
Epsztein, J., Brecht, M. & Lee, A.K. Intracellular determinants of hippocampal CA1 place and silent cell activity in a novel environment. Neuron 70, 109–120 (2011).
Cohen, J.D., Bolstad, M. & Lee, A.K. Experience-dependent shaping of hippocampal CA1 intracellular activity in novel and familiar environments. eLife 6, e23040 (2017).This study provides fundamental insight into the mechanisms of place cell formation. Using intracellular recordings from place cells in mice navigating in novel or familiar virtual environments, the authors show how membrane potential dynamics change during the development of novel place fields.
Silva, A.J., Zhou, Y., Rogerson, T., Shobe, J. & Balaji, J. Molecular and cellular approaches to memory allocation in neural circuits. Science 326, 391–395 (2009).
Zhou, Y. et al. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat. Neurosci. 12, 1438–1443 (2009).
Yiu, A.P. et al. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron 83, 722–735 (2014).
Rashid, A.J. et al. Competition between engrams influences fear memory formation and recall. Science 353, 383–387 (2016).
Rubin, A., Geva, N., Sheintuch, L. & Ziv, Y. Hippocampal ensemble dynamics timestamp events in long-term memory. eLife 4, e12247 (2015).This study reveals a temporal code for time-stamping events. By following the activity of populations of place cells over several weeks, the authors found that the timing of experience on a timescale of days can be decoded from CA1 population activity independently of context.
Cai, D.J. et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534, 115–118 (2016).This study tests hypothesized roles of excitability in memory allocation within CA1. Convergent evidence from imaging of place cell activity, anatomical tagging of activated cells, and behavioral experiments supports the idea that memories of events that are close in time are allocated to overlapping populations of neurons.
Kastellakis, G., Silva, A.J. & Poirazi, P. Linking memories across time via neuronal and dendritic overlaps in model neurons with active dendrites. Cell Rep. 17, 1491–1504 (2016).
Dudek, S.M. & Fields, R.D. Somatic action potentials are sufficient for late-phase LTP-related cell signaling. Proc. Natl. Acad. Sci. USA 99, 3962–3967 (2002).
Deisseroth, K., Bito, H. & Tsien, R.W. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron 16, 89–101 (1996).
Lopez de Armentia, M. et al. cAMP response element-binding protein-mediated gene expression increases the intrinsic excitability of CA1 pyramidal neurons. J. Neurosci. 27, 13909–13918 (2007).
Disterhoft, J.F. & Oh, M.M. Learning, aging and intrinsic neuronal plasticity. Trends Neurosci. 29, 587–599 (2006).
Lovett-Barron, M. et al. Dendritic inhibition in the hippocampus supports fear learning. Science 343, 857–863 (2014).
Stefanelli, T., Bertollini, C., Lüscher, C., Muller, D. & Mendez, P. Hippocampal somatostatin interneurons control the size of neuronal memory ensembles. Neuron 89, 1074–1085 (2016).
Mankin, E.A. et al. Neuronal code for extended time in the hippocampus. Proc. Natl. Acad. Sci. USA 109, 19462–19467 (2012).
Mankin, E.A., Diehl, G.W., Sparks, F.T., Leutgeb, S. & Leutgeb, J.K. Hippocampal CA2 activity patterns change over time to a larger extent than between spatial contexts. Neuron 85, 190–201 (2015).
Lipowsky, R., Gillessen, T. & Alzheimer, C. Dendritic Na+ channels amplify EPSPs in hippocampal CA1 pyramidal cells. J. Neurophysiol. 76, 2181–2191 (1996).
Magee, J.C. Dendritic lh normalizes temporal summation in hippocampal CA1 neurons. Nat. Neurosci. 2, 508–514 (1999).
Ngo-Anh, T.J. et al. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat. Neurosci. 8, 642–649 (2005).
Losonczy, A., Makara, J.K. & Magee, J.C. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature 452, 436–441 (2008).
Amaral, D.G. & Witter, M.P. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31, 571–591 (1989).
Freund, T.F. & Buzsáki, G. Interneurons of the hippocampus. Hippocampus 6, 347–470 (1996).
Middleton, S.J. & McHugh, T.J. Silencing CA3 disrupts temporal coding in the CA1 ensemble. Nat. Neurosci. 19, 945–951 (2016).
Schlesiger, M.I. et al. The medial entorhinal cortex is necessary for temporal organization of hippocampal neuronal activity. Nat. Neurosci. 18, 1123–1132 (2015).
Magee, J.C. Dendritic integration of excitatory synaptic input. Nat. Rev. Neurosci. 1, 181–190 (2000).
Nolan, M.F. et al. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell 119, 719–732 (2004).
Tsay, D., Dudman, J.T. & Siegelbaum, S.A. HCN1 channels constrain synaptically evoked Ca2+ spikes in distal dendrites of CA1 pyramidal neurons. Neuron 56, 1076–1089 (2007).
Cai, X. et al. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron 44, 351–364 (2004).
Lee, D., Lin, B.-J. & Lee, A.K. Hippocampal place fields emerge upon single-cell manipulation of excitability during behavior. Science 337, 849–853 (2012).This work reveals surprisingly nonlinear properties of place cells. The authors obtained intracellular recordings from hippocampal neurons in freely navigating mice. They show that silent cells without any spatial modulation of membrane potential can be turned into place cells by sustained depolarization.
Nevian, T., Larkum, M.E., Polsky, A. & Schiller, J. Properties of basal dendrites of layer 5 pyramidal neurons: a direct patch-clamp recording study. Nat. Neurosci. 10, 206–214 (2007).
Druckmann, S. et al. Structured synaptic connectivity between hippocampal regions. Neuron 81, 629–640 (2014).
Wilson, D.E., Whitney, D.E., Scholl, B. & Fitzpatrick, D. Orientation selectivity and the functional clustering of synaptic inputs in primary visual cortex. Nat. Neurosci. 19, 1003–1009 (2016).
Iacaruso, M.F., Gasler, I.T. & Hofer, S.B. Synaptic organization of visual space in primary visual cortex. Nature 547, 449–452 (2017).
Haider, B., Häusser, M. & Carandini, M. Inhibition dominates sensory responses in the awake cortex. Nature 493, 97–100 (2013).
Atallah, B.V., Bruns, W., Carandini, M. & Scanziani, M. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron 73, 159–170 (2012).
Atallah, B.V., Scanziani, M. & Carandini, M. Atallah et al. reply. Nature 508, E3 (2014).
Grienberger, C., Milstein, A.D., Bittner, K.C., Romani, S. & Magee, J.C. Inhibitory suppression of heterogeneously tuned excitation enhances spatial coding in CA1 place cells. Nat. Neurosci. 20, 417–426 (2017).Using optogenetic silencing of hippocampal interneurons in conjunction with intracellular recordings from place cells, the authors of this study addressed the role of inhibition in the shaping of place fields. They show that uniform inhibition can contribute to place field tuning, probably by suppressing firing outside of the place field.
Wilson, N.R., Runyan, C.A., Wang, F.L. & Sur, M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature 488, 343–348 (2012).
Lee, S.-H. et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature 488, 379–383 (2012).
Cottam, J.C.H., Smith, S.L. & Häusser, M. Target-specific effects of somatostatin-expressing interneurons on neocortical visual processing. J. Neurosci. 33, 19567–19578 (2013).
Royer, S. et al. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat. Neurosci. 15, 769–775 (2012).
Yoon, K. et al. Specific evidence of low-dimensional continuous attractor dynamics in grid cells. Nat. Neurosci. 16, 1077–1084 (2013).
Fyhn, M., Hafting, T., Treves, A., Moser, M.-B. & Moser, E.I. Hippocampal remapping and grid realignment in entorhinal cortex. Nature 446, 190–194 (2007).
Pastoll, H., Ramsden, H.L. & Nolan, M.F. Intrinsic electrophysiological properties of entorhinal cortex stellate cells and their contribution to grid cell firing fields. Front. Neural Circuits 6, 17 (2012).
Ray, S. et al. Grid-layout and theta-modulation of layer 2 pyramidal neurons in medial entorhinal cortex. Science 343, 891–896 (2014).
Couey, J.J. et al. Recurrent inhibitory circuitry as a mechanism for grid formation. Nat. Neurosci. 16, 318–324 (2013).
Pastoll, H., Solanka, L., van Rossum, M.C.W. & Nolan, M.F. Feedback inhibition enables θ-nested γ oscillations and grid firing fields. Neuron 77, 141–154 (2013).
Nolan, M.F., Dudman, J.T., Dodson, P.D. & Santoro, B. HCN1 channels control resting and active integrative properties of stellate cells from layer II of the entorhinal cortex. J. Neurosci. 27, 12440–12451 (2007).
Giocomo, L.M. et al. Grid cells use HCN1 channels for spatial scaling. Cell 147, 1159–1170 (2011).
Robinson, R.B. & Siegelbaum, S.A. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu. Rev. Physiol. 65, 453–480 (2003).
Garden, D.L.F., Dodson, P.D., O'Donnell, C., White, M.D. & Nolan, M.F. Tuning of synaptic integration in the medial entorhinal cortex to the organization of grid cell firing fields. Neuron 60, 875–889 (2008).
Giocomo, L.M., Zilli, E.A., Fransén, E. & Hasselmo, M.E. Temporal frequency of subthreshold oscillations scales with entorhinal grid cell field spacing. Science 315, 1719–1722 (2007).
Schmidt-Hieber, C. et al. Active dendritic integration as a mechanism for robust and precise grid cell firing. Nat. Neurosci. 20, 1114–1121 (2017).
Palmer, L.M. et al. NMDA spikes enhance action potential generation during sensory input. Nat. Neurosci. 17, 383–390 (2014).
Lavzin, M., Rapoport, S., Polsky, A., Garion, L. & Schiller, J. Nonlinear dendritic processing determines angular tuning of barrel cortex neurons in vivo. Nature 490, 397–401 (2012).
Smith, S.L., Smith, I.T., Branco, T. & Häusser, M. Dendritic spikes enhance stimulus selectivity in cortical neurons in vivo. Nature 503, 115–120 (2013).
Poirazi, P. & Mel, B.W. Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron 29, 779–796 (2001).
Buzsáki, G. & Moser, E.I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 16, 130–138 (2013).
Martin, S.J., Grimwood, P.D. & Morris, R.G. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 23, 649–711 (2000).
Bliss, T.V. & Collingridge, G.L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 (1993).
Morris, R.G., Anderson, E., Lynch, G.S. & Baudry, M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-d-aspartate receptor antagonist, AP5. Nature 319, 774–776 (1986).
Tsien, J.Z., Huerta, P.T. & Tonegawa, S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87, 1327–1338 (1996).
Kentros, C. et al. Abolition of long-term stability of new hippocampal place cell maps by NMDA receptor blockade. Science 280, 2121–2126 (1998).
McHugh, T.J., Blum, K.I., Tsien, J.Z., Tonegawa, S. & Wilson, M.A. Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice. Cell 87, 1339–1349 (1996).
Moosmang, S. et al. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J. Neurosci. 25, 9883–9892 (2005).
Sheffield, M.E.J. & Dombeck, D.A. Calcium transient prevalence across the dendritic arbour predicts place field properties. Nature 517, 200–204 (2015).
Takahashi, H. & Magee, J.C. Pathway interactions and synaptic plasticity in the dendritic tuft regions of CA1 pyramidal neurons. Neuron 62, 102–111 (2009).
Bittner, K.C. et al. Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nat. Neurosci. 18, 1133–1142 (2015).
Bittner, K.C., Milstein, A.D., Grienberger, C., Romani, S. & Magee, J.C. Behavioral time scale synaptic plasticity underlies CA1 place fields. Science 357, 1033–1036 (2017).This study reports an unusual form of synaptic plasticity in CA1 pyramidal cells, whereby a single pairing of pre- and postsynaptic activity within a time window of several seconds produces long-term potentiation of the synaptic response. Such a pairing paradigm may store events that occur at behavioral timescales.
Golding, N.L., Staff, N.P. & Spruston, N. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature 418, 326–331 (2002).
Dudman, J.T., Tsay, D. & Siegelbaum, S.A. A role for synaptic inputs at distal dendrites: instructive signals for hippocampal long-term plasticity. Neuron 56, 866–879 (2007).
Frank, L.M., Stanley, G.B. & Brown, E.N. Hippocampal plasticity across multiple days of exposure to novel environments. J. Neurosci. 24, 7681–7689 (2004).
Hill, A.J. First occurrence of hippocampal spatial firing in a new environment. Exp. Neurol. 62, 282–297 (1978).
Brandalise, F. & Gerber, U. Mossy fiber-evoked subthreshold responses induce timing-dependent plasticity at hippocampal CA3 recurrent synapses. Proc. Natl. Acad. Sci. USA 111, 4303–4308 (2014).
Brandalise, F., Carta, S., Helmchen, F., Lisman, J. & Gerber, U. Dendritic NMDA spikes are necessary for timing-dependent associative LTP in CA3 pyramidal cells. Nat. Commun. 7, 13480 (2016).
Lörincz, A., Notomi, T., Tamás, G., Shigemoto, R. & Nusser, Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat. Neurosci. 5, 1185–1193 (2002).
Hussaini, S.A., Kempadoo, K.A., Thuault, S.J., Siegelbaum, S.A. & Kandel, E.R. Increased size and stability of CA1 and CA3 place fields in HCN1 knockout mice. Neuron 72, 643–653 (2011).
Maroso, M. et al. Cannabinoid control of learning and memory through HCN channels. Neuron 89, 1059–1073 (2016).
Magee, J.C. Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat. Neurosci. 2, 848 (1999).
Buzsáki, G. Theta oscillations in the hippocampus. Neuron 33, 325–340 (2002).
O'Keefe, J. & Recce, M.L. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3, 317–330 (1993).
Bender, F. et al. Theta oscillations regulate the speed of locomotion via a hippocampus to lateral septum pathway. Nat. Commun. 6, 8521 (2015).
Freund, T.F. & Antal, M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature 336, 170–173 (1988).
Boyce, R., Glasgow, S.D., Williams, S. & Adamantidis, A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 352, 812–816 (2016).
Gonzalez-Sulser, A. et al. GABAergic projections from the medial septum selectively inhibit interneurons in the medial entorhinal cortex. J. Neurosci. 34, 16739–16743 (2014).
Tóth, K., Freund, T.F. & Miles, R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. J. Physiol. (Lond.) 500, 463–474 (1997).
Mizuseki, K., Sirota, A., Pastalkova, E. & Buzsáki, G. Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron 64, 267–280 (2009).
Pike, F.G. et al. Distinct frequency preferences of different types of rat hippocampal neurones in response to oscillatory input currents. J. Physiol. (Lond.) 529, 205–213 (2000).
Zemankovics, R., Káli, S., Paulsen, O., Freund, T.F. & Hájos, N. Differences in subthreshold resonance of hippocampal pyramidal cells and interneurons: the role of h-current and passive membrane characteristics. J. Physiol. (Lond.) 588, 2109–2132 (2010).
Leung, L.S. & Yu, H.W. Theta-frequency resonance in hippocampal CA1 neurons in vitro demonstrated by sinusoidal current injection. J. Neurophysiol. 79, 1592–1596 (1998).
Erchova, I., Kreck, G., Heinemann, U. & Herz, A.V.M. Dynamics of rat entorhinal cortex layer II and III cells: characteristics of membrane potential resonance at rest predict oscillation properties near threshold. J. Physiol. (Lond.) 560, 89–110 (2004).
Hu, H., Vervaeke, K. & Storm, J.F. Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na+ current in rat hippocampal pyramidal cells. J. Physiol. (Lond.) 545, 783–805 (2002).
Peters, H.C., Hu, H., Pongs, O., Storm, J.F. & Isbrandt, D. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat. Neurosci. 8, 51–60 (2005).
Borel, M., Guadagna, S., Jang, H.J., Kwag, J. & Paulsen, O. Frequency dependence of CA3 spike phase response arising from h-current properties. Front. Cell. Neurosci. 7, 263 (2013).
Ness, T.V., Remme, M.W.H. & Einevoll, G.T. Active subthreshold dendritic conductances shape the local field potential. J. Physiol. (Lond.) 594, 3809–3825 (2016).
Stark, E. et al. Inhibition-induced theta resonance in cortical circuits. Neuron 80, 1263–1276 (2013).The experiments in this study provide evidence for theta-frequency resonance in behaving animals. Intriguingly, resonant responses of CA1 pyramidal cells were observed in response to the activation of PV interneurons, but not after direct activation.
Alonso, A. & Llinás, R.R. Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature 342, 175–177 (1989).
Leung, L.W. & Yim, C.Y. Intrinsic membrane potential oscillations in hippocampal neurons in vitro. Brain Res. 553, 261–274 (1991).
Fernandez, F.R. & White, J.A. Artificial synaptic conductances reduce subthreshold oscillations and periodic firing in stellate cells of the entorhinal cortex. J. Neurosci. 28, 3790–3803 (2008).
Jaramillo, J. & Kempter, R. Phase precession: a neural code underlying episodic memory? Curr. Opin. Neurobiol. 43, 130–138 (2017).
Leung, L.S. A model of intracellular θ phase precession dependent on intrinsic subthreshold membrane currents. J. Neurosci. 31, 12282–12296 (2011).
Harris, K.D. et al. Spike train dynamics predicts theta-related phase precession in hippocampal pyramidal cells. Nature 417, 738–741 (2002).
Magee, J.C. Dendritic mechanisms of phase precession in hippocampal CA1 pyramidal neurons. J. Neurophysiol. 86, 528–532 (2001).
Mehta, M.R., Lee, A.K. & Wilson, M.A. Role of experience and oscillations in transforming a rate code into a temporal code. Nature 417, 741–746 (2002).
Mehta, M.R., Quirk, M.C. & Wilson, M.A. Experience-dependent asymmetric shape of hippocampal receptive fields. Neuron 25, 707–715 (2000).
Chadwick, A., van Rossum, M.C.W. & Nolan, M.F. Independent theta phase coding accounts for CA1 population sequences and enables flexible remapping. eLife 4, e03542 (2015).
Eggink, H., Mertens, P., Storm, E. & Giocomo, L.M. Hyperpolarization-activated cyclic nucleotide-gated 1 independent grid cell-phase precession in mice. Hippocampus 24, 249–256 (2014).
Kim, S., Guzman, S.J., Hu, H. & Jonas, P. Active dendrites support efficient initiation of dendritic spikes in hippocampal CA3 pyramidal neurons. Nat. Neurosci. 15, 600–606 (2012).
Makara, J.K. & Magee, J.C. Variable dendritic integration in hippocampal CA3 pyramidal neurons. Neuron 80, 1438–1450 (2013).
Krueppel, R., Remy, S. & Beck, H. Dendritic integration in hippocampal dentate granule cells. Neuron 71, 512–528 (2011).
Branco, T., Clark, B.A. & Häusser, M. Dendritic discrimination of temporal input sequences in cortical neurons. Science 329, 1671–1675 (2010).
Stocca, G., Schmidt-Hieber, C. & Bischofberger, J. Differential dendritic Ca2+ signalling in young and mature hippocampal granule cells. J. Physiol. (Lond.) 586, 3795–3811 (2008).
McHugh, T.J. et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317, 94–99 (2007).
Chavlis, S., Petrantonakis, P.C. & Poirazi, P. Dendrites of dentate gyrus granule cells contribute to pattern separation by controlling sparsity. Hippocampus 27, 89–110 (2017).
Schmidt-Hieber, C., Jonas, P. & Bischofberger, J. Subthreshold dendritic signal processing and coincidence detection in dentate gyrus granule cells. J. Neurosci. 27, 8430–8441 (2007).
Valero, M. et al. Determinants of different deep and superficial CA1 pyramidal cell dynamics during sharp-wave ripples. Nat. Neurosci. 18, 1281–1290 (2015).
Gan, J., Weng, S.-M., Pernía-Andrade, A.J., Csicsvari, J. & Jonas, P. Phase-locked inhibition, but not excitation, underlies hippocampal ripple oscillations in awake mice in vivo. Neuron 93, 308–314 (2017).
Cobb, S. & Lawrence, J.J. Neuromodulation of hippocampal cells and circuits. In Hippocampal Microcircuits: A Computational Modeler's Resource Book (eds. Cutsuridis, V., Graham, B.P., Cobb, S. & Vida, I.) 187–246 (Springer, 2010).
Marder, E. Neuromodulation of neuronal circuits: back to the future. Neuron 76, 1–11 (2012).
Martinello, K. et al. Cholinergic afferent stimulation induces axonal function plasticity in adult hippocampal granule cells. Neuron 85, 346–363 (2015).
Hasselmo, M.E., Hay, J., Ilyn, M. & Gorchetchnikov, A. Neuromodulation, theta rhythm and rat spatial navigation. Neural Netw. 15, 689–707 (2002).
Kramer, R.H., Mourot, A. & Adesnik, H. Optogenetic pharmacology for control of native neuronal signaling proteins. Nat. Neurosci. 16, 816–823 (2013).
Hamel, E.J.O., Grewe, B.F., Parker, J.G. & Schnitzer, M.J. Cellular level brain imaging in behaving mammals: an engineering approach. Neuron 86, 140–159 (2015).
Gong, Y. et al. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science 350, 1361–1366 (2015).
Koenig, J., Linder, A.N., Leutgeb, J.K. & Leutgeb, S. The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science 332, 592–595 (2011).
Acknowledgements
We thank N. Rochefort and G. Sürmeli for comments on the manuscript, A. Lee for sharing data before publication (ref. 26), and G. Buzsáki and E. Stark for sharing material for the generation of figures. This work was funded in part by the BBSRC (grants BB/M025454/1 and BB/L010496/1 to M.F.N.), the Human Frontiers Science Program (grant RGP0062/2014 to M.F.N.), the Wellcome Trust (grant 200855/Z/16/Z to M.F.N.), the Simons Initiative for the Developing Brain (M.F.N.) and the ERC (grant StG 678790 NEWRON to C.S.-H.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Schmidt-Hieber, C., Nolan, M. Synaptic integrative mechanisms for spatial cognition. Nat Neurosci 20, 1483–1492 (2017). https://doi.org/10.1038/nn.4652
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4652
This article is cited by
-
Brain–heart interaction disruption in major depressive disorder: disturbed rhythm modulation of the cardiac cycle on brain transient theta bursts
European Archives of Psychiatry and Clinical Neuroscience (2024)
-
A synaptic signal for novelty processing in the hippocampus
Nature Communications (2022)
-
Multimodal in vivo brain electrophysiology with integrated glass microelectrodes
Nature Biomedical Engineering (2019)
-
Enhancement of dendritic persistent Na+ currents by mGluR5 leads to an advancement of spike timing with an increase in temporal precision
Molecular Brain (2018)