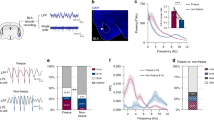
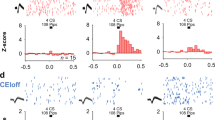
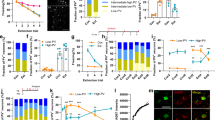
Abstract
The mammalian brain contains dedicated circuits for both the learned expression and suppression of fear. These circuits require precise coordination to facilitate the appropriate expression of fear behavior, but the mechanisms underlying this coordination remain unclear. Using a combination of chemogenetics, activity-based neuronal-ensemble labeling and in vivo electrophysiology, we found that fear extinction learning confers on parvalbumin-expressing (PV) interneurons in the basolateral amygdala (BLA) a dedicated role in the selective suppression of a previously encoded fear memory and BLA fear-encoding neurons. In addition, following extinction learning, PV interneurons enable a competing interaction between a 6–12 Hz oscillation and a fear-associated 3–6 Hz oscillation within the BLA. Loss of this competition increases a 3–6 Hz oscillatory signature, with BLA→medial prefrontal cortex directionality signaling the recurrence of fear expression. The discovery of cellular and oscillatory substrates of fear extinction learning that critically depend on BLA PV interneurons could inform therapies aimed at preventing the pathological recurrence of fear following extinction learning.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Milad, M.R. & Quirk, G.J. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 63, 129–151 (2012).
Kearns, M.C., Ressler, K.J., Zatzick, D. & Rothbaum, B.O. Early interventions for PTSD: a review. Depress. Anxiety 29, 833–842 (2012).
Foa, E.B. Prolonged exposure therapy: past, present, and future. Depress. Anxiety 28, 1043–1047 (2011).
Myers, K.M. & Davis, M. Mechanisms of fear extinction. Mol. Psychiatry 12, 120–150 (2007).
Tovote, P., Fadok, J.P. & Lüthi, A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 16, 317–331 (2015).
Reijmers, L.G., Perkins, B.L., Matsuo, N. & Mayford, M. Localization of a stable neural correlate of associative memory. Science 317, 1230–1233 (2007).
Trouche, S., Sasaki, J.M., Tu, T. & Reijmers, L.G. Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron 80, 1054–1065 (2013).
Wolff, S.B.E. et al. Amygdala interneuron subtypes control fear learning through disinhibition. Nature 509, 453–458 (2014).
Armbruster, B.N., Li, X., Pausch, M.H., Herlitze, S. & Roth, B.L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. USA 104, 5163–5168 (2007).
Vereczki, V.K. et al. Synaptic organization of perisomatic GABAergic inputs onto the principal cells of the mouse basolateral amygdala. Front. Neuroanat. 10, 20 (2016).
Seidenbecher, T., Laxmi, T.R., Stork, O. & Pape, H.C. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science 301, 846–850 (2003).
Karalis, N. et al. 4-Hz oscillations synchronize prefrontal-amygdala circuits during fear behavior. Nat. Neurosci. 19, 605–612 (2016).
Wall, N.R., Wickersham, I.R., Cetin, A., De La Parra, M. & Callaway, E.M. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proc. Natl. Acad. Sci. USA 107, 21848–21853 (2010).
Giustino, T.F. & Maren, S. The role of the medial prefrontal cortex in the conditioning and extinction of fear. Front. Behav. Neurosci. 9, 298 (2015).
Adhikari, A., Sigurdsson, T., Topiwala, M.A. & Gordon, J.A. Cross-correlation of instantaneous amplitudes of field potential oscillations: a straightforward method to estimate the directionality and lag between brain areas. J. Neurosci. Methods 191, 191–200 (2010).
Donato, F., Rompani, S.B. & Caroni, P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 504, 272–276 (2013).
Lucas, E.K., Jegarl, A.M., Morishita, H. & Clem, R.L. Multimodal and site-specific plasticity of amygdala parvalbumin interneurons after fear learning. Neuron 91, 629–643 (2016).
Rashid, A.J. et al. Competition between engrams influences fear memory formation and recall. Science 353, 383–387 (2016).
de Almeida, L., Idiart, M. & Lisman, J.E. A second function of gamma frequency oscillations: an E%-max winner-take-all mechanism selects which cells fire. J. Neurosci. 29, 7497–7503 (2009).
Roux, L. & Buzsáki, G. Tasks for inhibitory interneurons in intact brain circuits. Neuropharmacology 88, 10–23 (2015).
Smith, Y., Paré, J.-F. & Paré, D. Differential innervation of parvalbumin-immunoreactive interneurons of the basolateral amygdaloid complex by cortical and intrinsic inputs. J. Comp. Neurol. 416, 496–508 (2000).
Hübner, C., Bosch, D., Gall, A., Lüthi, A. & Ehrlich, I. Ex vivo dissection of optogenetically activated mPFC and hippocampal inputs to neurons in the basolateral amygdala: implications for fear and emotional memory. Front. Behav. Neurosci. 8, 64 (2014).
Kim, J., Pignatelli, M., Xu, S., Itohara, S. & Tonegawa, S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat. Neurosci. 19, 1636–1646 (2016).
Little, J.P. & Carter, A.G. Synaptic mechanisms underlying strong reciprocal connectivity between the medial prefrontal cortex and basolateral amygdala. J. Neurosci. 33, 15333–15342 (2013).
McGarry, L.M. & Carter, A.G. Inhibitory gating of basolateral amygdala inputs to the prefrontal cortex. J. Neurosci. 36, 9391–9406 (2016).
Quirk, G.J., Likhtik, E., Pelletier, J.G. & Paré, D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J. Neurosci. 23, 8800–8807 (2003).
Royer, S. & Paré, D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience 115, 455–462 (2002).
Likhtik, E., Popa, D., Apergis-Schoute, J., Fidacaro, G.A. & Paré, D. Amygdala intercalated neurons are required for expression of fear extinction. Nature 454, 642–645 (2008).
Amano, T., Unal, C.T. & Paré, D. Synaptic correlates of fear extinction in the amygdala. Nat. Neurosci. 13, 489–494 (2010).
Jüngling, K. et al. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron 59, 298–310 (2008).
Maren, S., Phan, K.L. & Liberzon, I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 14, 417–428 (2013).
Redondo, R.L. et al. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513, 426–430 (2014).
Xu, C. et al. Distinct hippocampal pathways mediate dissociable roles of context in memory retrieval. Cell 167, 961–972.e16 (2016).
Bazelot, M. et al. Hippocampal theta input to the amygdala shapes feedforward inhibition to gate heterosynaptic plasticity. Neuron 87, 1290–1303 (2015).
Bienvenu, T.C.M., Busti, D., Magill, P.J., Ferraguti, F. & Capogna, M. Cell-type-specific recruitment of amygdala interneurons to hippocampal theta rhythm and noxious stimuli in vivo. Neuron 74, 1059–1074 (2012).
Orsini, C.A., Kim, J.H., Knapska, E. & Maren, S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J. Neurosci. 31, 17269–17277 (2011).
Sotres-Bayon, F., Sierra-Mercado, D., Pardilla-Delgado, E. & Quirk, G.J. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76, 804–812 (2012).
Buzsáki, G. Theta oscillations in the hippocampus. Neuron 33, 325–340 (2002).
Ryan, S.J. et al. Spike-timing precision and neuronal synchrony are enhanced by an interaction between synaptic inhibition and membrane oscillations in the amygdala. PLoS One 7, e35320 (2012).
Herry, C. et al. Switching on and off fear by distinct neuronal circuits. Nature 454, 600–606 (2008).
Paré, D. & Gaudreau, H. Projection cells and interneurons of the lateral and basolateral amygdala: distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. J. Neurosci. 16, 3334–3350 (1996).
Popa, D., Duvarci, S., Popescu, A.T., Léna, C. & Paré, D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc. Natl. Acad. Sci. USA 107, 6516–6519 (2010).
Likhtik, E., Stujenske, J.M., Topiwala, M.A., Harris, A.Z. & Gordon, J.A. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat. Neurosci. 17, 106–113 (2014).
Stujenske, J.M., Likhtik, E., Topiwala, M.A. & Gordon, J.A. Fear and safety engage competing patterns of theta-gamma coupling in the basolateral amygdala. Neuron 83, 919–933 (2014).
Klavir, O., Prigge, M., Sarel, A., Paz, R. & Yizhar, O. Manipulating fear associations via optogenetic modulation of amygdala inputs to prefrontal cortex. Nat. Neurosci. 20, 836–844 (2017).
Burgos-Robles, A. et al. Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat. Neurosci. 20, 824–835 (2017).
Schiller, D. et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463, 49–53 (2010).
Agren, T. et al. Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science 337, 1550–1552 (2012).
Björkstrand, J. et al. Disrupting reconsolidation attenuates long-term fear memory in the human amygdala and facilitates approach behavior. Curr. Biol. 26, 2690–2695 (2016).
Delorme, A. et al. EEGLAB, SIFT, NFT, BCILAB, and ERICA: new tools for advanced EEG processing. Comput. Intell. Neurosci. 2011, 130714 (2011).
Acknowledgements
We thank B. Roth (UNC Vector Core) and E. Callaway (Salk Institute) for reagents. We thank A. Poulopoulos and T. Papouin for discussions and critical reading of the manuscript. We thank J. Sasaki Russell and S. Viola for technical assistance. This work was supported in part by grants to L.G.R. (NIH R01 MH104589) and J.M. (NIH R01 NS102937), and by the Tufts Center for Neuroscience Research (NIH P30 NS047243). P.D. was supported by the Synapse Neurobiology Training Program (NIH T32 NS061764) and the Medical Scientist Training Program at Tufts University (NIH T32 GM008448).
Author information
Authors and Affiliations
Contributions
P.D., J.M., and L.G.R. conceived and designed the experiments. P.D. and Y.Z. executed the experiments. P.D., Y.Z., and L.G.R. analyzed the experiments. P.D. and L.G.R. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Selective expression of hM4Di-mCherry in BLA PV interneurons mediates behavioral effect.
a) Representative 10x confocal z-stack of BLA showing selective expression of hM4Di-mCherry in parvalbumin-positive (PV) interneurons (red: mCherry, green: parvalbumin). b) Quantification of % overlap between hM4Di-mCHerry and PV (n = 10 mice). c-f) The number of tagged GFP+ neurons did not differ between VEH and CNO injected mice (c, unpaired t-test: t(13) = 0.7906, P = 0.4433, VEH n = 7 mice, CNO n = 8 mice; d, unpaired t-test: t(10) = 0.2709, P = 0.7920, VEH n = 7 mice, CNO n = 5 mice; C3 L2-3, Mann-Whitney test: U = 21, P = 0.8981, VEH n = 5 mice, CNO n = 9 mice; e, L5-6, Mann-Whitney test: U = 21, P = 0.8981, VEH n = 5 mice, CNO n = 9 mice; f, L2-3, Mann-Whitney test: U = 20, P = 0.7972, VEH n = 5 mice, CNO n = 9 mice; C4 L5-6, Mann-Whitney test: U = 22, P > 0.9999, VEH n = 5 mice, CNO n = 9 mice;). g) Mice exhibit increasing freezing levels as conditioning progresses on day 1, and decreasing freezing levels as extinction progresses on days 2-3 (Wilcoxon matched-pairs FC1 versus EXT1: W = 435, P < 0.0001, n = 29 mice; Wilcoxon matched-pairs EXT1 versus EXT8: W = -435, P < 0.0001, n = 29 mice). h) Mice freeze more during retrieval after CNO injection regardless of order of trials (CNO first, Wilcoxon matched-pairs: W = -75, P = 0.0015, n = 13 mice; VEH first, Wilcoxon matched-pairs: W = 77, P = 0.0264, n = 16 mice). i) CNO has no effect on behavior in animals not expressing hM4Di (SHAM injection) (Wilcoxon matched-pairs: W = 7, P = 0.7344, n = 9 mice). j) Mice exhibit increased freezing levels after CNO injection compared to VEH injection in the EXT-tagged group (Wilcoxon matched-pairs: W = 75, P = 0.0059, n = 13 mice). All box plot graphs show median (line inside box), 25% and 75% percentiles (box edges), and minimum and maximum values (error bars).
Supplementary Figure 2 Perisomatic analysis of BLA ensembles.
a) Example 40x confocal z-stack showing extraction of hM4Di-mCHerry perisomatic signal (middle) from nuclear ZIF signal (right) using DAPI mask. INSET: Example GFP+ neuron displayed in left panels. b) Representative ZIF+ and ZIF- nuclei in BLA of CNO- and VEH-injected mice demonstrating relationship between perisomatic puncta and ZIF expression. c) The amount of perisomatic mCherry around tagged GFP+ neurons, normalized within each mouse to perisomatic mCherry around GFP- neurons, was similar in FC-tagged and EXT tagged mice (unpaired t-test: t(16) = 0.04475, P = 0.9649, FC-tagged n = 8 mice, EXT-tagged n = 10 mice). Box plot graph shows median (line inside box), 25% and 75% percentiles (box edges), and minimum and maximum values (error bars).
Supplementary Figure 3 Oscillatory states following fear conditioning and following fear extinction.
a) Location of electrode tips in BLA (left), and mPFC (right), with example images of nissl-stained tissue showing electrode sites. b) Freezing data (minutes 2-3) from fear conditioning and extinction trials of mice used for LFP recordings (Wilcoxon matched-pairs FC1 versus EXT1: W = 64, P = 0.0020, n = 11 mice). c) Averaged power spectra across freezing and non-freezing bouts during EXT1 trials (BLA n = 11, mPFC n = 9; shaded bands mark standard error of mean). d) Averaged power spectra of post-extinction retrieval trials following vehicle or CNO injection (BLA n = 11, mPFC n = 9; shaded bands mark standard error of mean). e) Example of a VEH injected mouse demonstrating a correlation between BLA 3-6 Hz / 6-12 Hz power ratio and freezing during 12 time bins within a post-extinction retrieval trial (linear regression: F(1,10) = 19.68, P = 0.0013, n = 12 bins of 20 seconds each). f) The averaged within-trial Pearson’s correlation coefficients, calculated as in e), was significantly higher than zero in the VEH and CNO groups, but not in the EXT1 group (EXT1 Wilcoxon Signed Rank Test with theoretical median = 0: W = 13, P = 0.4961, n = 9 mice; VEH Wilcoxon Signed Rank Test with theoretical median = 0: W = 30, P = 0.0391, n = 8 mice; CNO Wilcoxon Signed Rank Test with theoretical median = 0: W = 55, P = 0.0020, n = 10 mice). g) CNO increases 3-6 / 6-12 Hz power ratio within bouts of freezing in both BLA (Wilcoxon matched-pairs: W = 66, P = 0.0010, n = 11), and in mPFC (Wilcoxon matched-pairs: W = 45, P = 0.0039, n = 9). h) Following fear conditioning, but before extinction takes effect (EXT1 trial), there is a trend for a larger mPFC lead in the 3-6 Hz band during the first 5 seconds following onset of freezing (3-6 Hz paired t-test: t(20) = 1.825, P = 0.0830, n = 21 mice; 6-12 Hz paired t-test: t(20) = 0.5692, P = 0.5756, n = 21 mice). i) Following fear conditioning, but before extinction takes effect (EXT1 trial), there is a trend for an increased probability that mPFC will lead BLA in the 3-6 Hz band, but not in the 6-12 Hz band (3-6 Hz Wilcoxon matched-pairs: W = 19, P = 0.0625, n = 8; 6-12 Hz Wilcoxon matched-pairs: W = -10, P = 0.1250, n = 8). All box plot graphs show median (line inside box), 25% and 75% percentiles (box edges), and minimum and maximum values (error bars).
Supplementary Figure 4 Example LFP traces filtered at different frequency ranges.
a) Example traces collected in the BLA during the first extinction trial on day 2 (EXT1), with red bar indicating freezing epochs. b) Example traces collected in the BLA during the first extinction trial on day 2 (EXT1), with red bar indicating freezing epochs. c) Example traces simultaneously collected in the BLA and mPFC during a post-extinction retrieval trial, with red bar indicating freezing epoch.
Supplementary Figure 5 Cross-correlation between 3–6 Hz and 6–12 Hz does not correlate with 6–12 Hz power.
a) Data from 10s bins from an example mouse demonstrating lack of correlation between 6-12 Hz power and 3-6 Hz: 6-12 Hz cross-correlation coefficients (VEH Pearson r = -0.08202, P = 0.8105, n = 11 bins of 10 seconds each; CNO Pearson r = 0.1049, P = 0.7731, n = 10 bins of 10 seconds each). b) Averaged Pearson’s correlation coefficients, calculated as in a), do not differ from zero in the VEH and CNO groups (VEH Wilcoxon Signed Rank Test with theoretical median = 0: W = -25, P = 0.2227, n = 10 mice; CNO Wilcoxon Signed Rank Test with theoretical median = 0: W = 7, P = 0.7520, n = 10 mice). c) Data from same analysis as in a) normalized to within-trial values and pooled together from all retrieval trials (VEH, linear regression: F(1,101) = 0.7014, R^2 = 0.0069, P = 0.4043, n = 103 bins of 10 sec each from 11 mice; CNO, linear regression: F(1,95) = 0.08185, R^2=0.0008, P = 0.7754, n = 97 bins of 10 sec each from 11 mice). Box plot graph shows median (line inside box), 25% and 75% percentiles (box edges), and minimum and maximum values (error bars).
Supplementary Figure 6 Granger causality analysis supports BLA→mPFC directionality in the 3–6 Hz range during post-extinction freezing.
a-b) Examples of Granger causality analysis for two post-extinction retrieval trials of the same mouse (a), first 2 minutes of the retrieval trial following vehicle injection; b), first 2 minutes of the retrieval trial following CNO injection; red boxes: periods of >50% freezing per bin; nDTF: normalized Directed Transfer Function). c) Granger causality when mice were freezing during post-extinction retrieval was significantly higher in BLA→mPFC than mPFC→BLA direction in the 4-8 Hz range (n = 7 mice; repeated measures two-way ANOVA: frequency F(14,84) = 4.889, P < 0.0001, direction F (1,6) = 10.34, P = 0.0182, frequency x direction F (14,84) = 2.947, P = 0.0011; Sidak's multiple comparisons tests used for comparing BLA→mPFC versus mPFC→BLA direction at single frequencies; shaded bands mark standard error of mean). d) Granger causality when mice were not freezing during post-extinction retrieval was significantly higher in BLA→mPFC than mPFC→BLA direction in the 5-8 Hz range (n = 7 mice; repeated measures two-way ANOVA: frequency F (14,84) = 3.918, P < 0.0001, direction F (1,6) = 4.796, P = 0.0711, frequency x direction F (14,84) = 2.725, P = 0.0024; Sidak's multiple comparisons tests used for comparing BLA→mPFC versus mPFC→BLA direction at single frequencies; shaded bands mark standard error of mean). e) During freezing epochs, but not during no freezing epochs, BLA→mPFC directionality was stronger in the 3-6 Hz range than in the 6-12 Hz range (n = 7 mice; freezing: paired t-test, t(6) = 4.83, P = 0.0029; no freezing: paired t-test, t(6) = 1.776, P = 0.1260). f) Freezing was associated with a higher relative BLA lead at 4 Hz (n = 7 mice; repeated measures two-way ANOVA: frequency F (14,84) = 3.167, P = 0.0005, freezing F (1,6) = 1.578, P = 0.2557, frequency x freezing F (14,84) = 2.089, P = 0.0203; Sidak's multiple comparisons tests used for comparing freezing versus no freezing at single frequencies; shaded bands mark standard error of mean). g) Freezing was associated with a higher relative 3-6 Hz BLA lead (n = 7 mice: Wilcoxon matched-pairs: W = -26, P = 0.0313). h) The CNO-induced increase in freezing correlated with the CNO-induced increase in relative 3-6 Hz BLA lead (n = 9 mice; linear regression: F(1,7) = 16.91, P = 0.0045). **** P < 0.0001, *** P < 0.001, ** P < 0.01, * P < 0.05. All box plot graphs show median (line inside box), 25% and 75% percentiles (box edges), and minimum and maximum values (error bars).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 1279 kb)
Rights and permissions
About this article
Cite this article
Davis, P., Zaki, Y., Maguire, J. et al. Cellular and oscillatory substrates of fear extinction learning. Nat Neurosci 20, 1624–1633 (2017). https://doi.org/10.1038/nn.4651
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4651
This article is cited by
-
Spatial transcriptomics reveal neuron–astrocyte synergy in long-term memory
Nature (2024)
-
Locus coeruleus input-modulated reactivation of dentate gyrus opioid-withdrawal engrams promotes extinction
Neuropsychopharmacology (2023)
-
The cerebellum regulates fear extinction through thalamo-prefrontal cortex interactions in male mice
Nature Communications (2023)
-
Thalamic nucleus reuniens coordinates prefrontal-hippocampal synchrony to suppress extinguished fear
Nature Communications (2023)
-
Nigrostriatal dopamine modulates the striatal-amygdala pathway in auditory fear conditioning
Nature Communications (2023)