Abstract
Blood flow into the brain is dynamically regulated to satisfy the changing metabolic requirements of neurons, but how this is accomplished has remained unclear. Here we demonstrate a central role for capillary endothelial cells in sensing neural activity and communicating it to upstream arterioles in the form of an electrical vasodilatory signal. We further demonstrate that this signal is initiated by extracellular K+ —a byproduct of neural activity—which activates capillary endothelial cell inward-rectifier K+ (KIR2.1) channels to produce a rapidly propagating retrograde hyperpolarization that causes upstream arteriolar dilation, increasing blood flow into the capillary bed. Our results establish brain capillaries as an active sensory web that converts changes in external K+ into rapid, 'inside-out' electrical signaling to direct blood flow to active brain regions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Iadecola, C. & Nedergaard, M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 10, 1369–1376 (2007).
Uhlirova, H. et al. Cell type specificity of neurovascular coupling in cerebral cortex. eLife 5, e14315 (2016).
Attwell, D. et al. Glial and neuronal control of brain blood flow. Nature 468, 232–243 (2010).
Nishimura, N., Schaffer, C.B., Friedman, B., Lyden, P.D. & Kleinfeld, D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc. Natl. Acad. Sci. USA 104, 365–370 (2007).
Blinder, P. et al. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat. Neurosci. 16, 889–897 (2013).
Beach, J.M., McGahren, E.D. & Duling, B.R. Capillaries and arterioles are electrically coupled in hamster cheek pouch. Am. J. Physiol. 275, H1489–H1496 (1998).
Aydin, F., Rosenblum, W.I. & Povlishock, J.T. Myoendothelial junctions in human brain arterioles. Stroke 22, 1592–1597 (1991).
Nelson, M.T., Patlak, J.B., Worley, J.F. & Standen, N.B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am. J. Physiol. 259, C3–C18 (1990).
Ballanyi, K., Doutheil, J. & Brockhaus, J. Membrane potentials and microenvironment of rat dorsal vagal cells in vitro during energy depletion. J. Physiol. (Lond.) 495, 769–784 (1996).
Longden, T.A. & Nelson, M.T. Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation 22, 183–196 (2015).
Quayle, J.M., Nelson, M.T. & Standen, N.B. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol. Rev. 77, 1165–1232 (1997).
Filosa, J.A. et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat. Neurosci. 9, 1397–1403 (2006).
Ledoux, J. et al. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc. Natl. Acad. Sci. USA 105, 9627–9632 (2008).
Quayle, J.M., McCarron, J.G., Brayden, J.E. & Nelson, M.T. Inward rectifier K+ currents in smooth muscle cells from rat resistance-sized cerebral arteries. Am. J. Physiol. 265, C1363–C1370 (1993).
Hibino, H. et al. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol. Rev. 90, 291–366 (2010).
Zaritsky, J.J., Eckman, D.M., Wellman, G.C., Nelson, M.T. & Schwarz, T.L. Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K(+) current in K(+)-mediated vasodilation. Circ. Res. 87, 160–166 (2000).
Ye, W. et al. The K+ channel KIR2.1 functions in tandem with proton influx to mediate sour taste transduction. Proc. Natl. Acad. Sci. USA 113, E229–E238 (2016).
Sonkusare, S.K., Dalsgaard, T., Bonev, A.D. & Nelson, M.T. Inward rectifier potassium (Kir2.1) channels as end-stage boosters of endothelium-dependent vasodilators. J. Physiol. (Lond.) 594, 3271–3285 (2016).
Ledoux, J., Werner, M.E., Brayden, J.E. & Nelson, M.T. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 21, 69–78 (2006).
Strøbaek, D. et al. Activation of human IK and SK Ca2+ -activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime). Biochim. Biophys. Acta 1665, 1–5 (2004).
Dabertrand, F. et al. Potassium channelopathy-like defect underlies early-stage cerebrovascular dysfunction in a genetic model of small vessel disease. Proc. Natl. Acad. Sci. USA 112, E796–E805 (2015).
Dabertrand, F. et al. Prostaglandin E2, a postulated astrocyte-derived neurovascular coupling agent, constricts rather than dilates parenchymal arterioles. J. Cereb. Blood Flow Metab. 33, 479–482 (2013).
Dabertrand, F., Nelson, M.T. & Brayden, J.E. Acidosis dilates brain parenchymal arterioles by conversion of calcium waves to sparks to activate BK channels. Circ. Res. 110, 285–294 (2012).
Hannah, R.M., Dunn, K.M., Bonev, A.D. & Nelson, M.T. Endothelial SK(Ca) and IK(Ca) channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J. Cereb. Blood Flow Metab. 31, 1175–1186 (2011).
Nystoriak, M.A. et al. Fundamental increase in pressure-dependent constriction of brain parenchymal arterioles from subarachnoid hemorrhage model rats due to membrane depolarization. Am. J. Physiol. Heart Circ. Physiol. 300, H803–H812 (2011).
Knot, H.J., Zimmermann, P.A. & Nelson, M.T. Extracellular K(+)-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K(+) channels. J. Physiol. (Lond.) 492, 419–430 (1996).
Girouard, H. et al. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc. Natl. Acad. Sci. USA 107, 3811–3816 (2010).
Ayata, C. & Lauritzen, M. Spreading depression, spreading depolarizations, and the cerebral vasculature. Physiol. Rev. 95, 953–993 (2015).
Toussay, X., Basu, K., Lacoste, B. & Hamel, E. Locus coeruleus stimulation recruits a broad cortical neuronal network and increases cortical perfusion. J. Neurosci. 33, 3390–3401 (2013).
Vetri, F., Xu, H., Paisansathan, C. & Pelligrino, D.A. Impairment of neurovascular coupling in type 1 diabetes mellitus in rats is linked to PKC modulation of BK(Ca) and Kir channels. Am. J. Physiol. Heart Circ. Physiol. 302, H1274–H1284 (2012).
Masamoto, K. et al. Unveiling astrocytic control of cerebral blood flow with optogenetics. Sci. Rep. 5, 11455 (2015).
Leithner, C. et al. Pharmacological uncoupling of activation induced increases in CBF and CMRO2. J. Cereb. Blood Flow Metab. 30, 311–322 (2010).
Shih, A.Y. et al. Robust and fragile aspects of cortical blood flow in relation to the underlying angioarchitecture. Microcirculation 22, 204–218 (2015).
García-Amado, M. & Prensa, L. Stereological analysis of neuron, glial and endothelial cell numbers in the human amygdaloid complex. PLoS One 7, e38692 (2012).
Tsai, P.S. et al. Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of nuclei and vessels. J. Neurosci. 29, 14553–14570 (2009).
Simard, M., Arcuino, G., Takano, T., Liu, Q.S. & Nedergaard, M. Signaling at the gliovascular interface. J. Neurosci. 23, 9254–9262 (2003).
Cauli, B. et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J. Neurosci. 24, 8940–8949 (2004).
Zhang, T., Wu, D.M., Xu, G.-Z. & Puro, D.G. The electrotonic architecture of the retinal microvasculature: modulation by angiotensin II. J. Physiol. (Lond.) 589, 2383–2399 (2011).
Figueroa, X.F. & Duling, B.R. Gap junctions in the control of vascular function. Antioxid. Redox Signal. 11, 251–266 (2009).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Kuschinsky, W., Wahl, M., Bosse, O. & Thurau, K. Perivascular potassium and pH as determinants of local pial arterial diameter in cats. A microapplication study. Circ. Res. 31, 240–247 (1972).
Daut, J., Standen, N.B. & Nelson, M.T. The role of the membrane potential of endothelial and smooth muscle cells in the regulation of coronary blood flow. J. Cardiovasc. Electrophysiol. 5, 154–181 (1994).
Hall, C.N. et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55–60 (2014).
Wu, D.M., Minami, M., Kawamura, H. & Puro, D.G. Electrotonic transmission within pericyte-containing retinal microvessels. Microcirculation 13, 353–363 (2006).
Matsushita, K. & Puro, D.G. Topographical heterogeneity of K(IR) currents in pericyte-containing microvessels of the rat retina: effect of diabetes. J. Physiol. (Lond.) 573, 483–495 (2006).
Wei, H.S. et al. Erythrocytes are oxygen-sensing regulators of the cerebral microcirculation. Neuron 91, 851–862 (2016).
Chen, B.R., Kozberg, M.G., Bouchard, M.B., Shaik, M.A. & Hillman, E.M.C. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J. Am. Heart Assoc. 3, e000787 (2014).
Westcott, E.B., Goodwin, E.L., Segal, S.S. & Jackson, W.F. Function and expression of ryanodine receptors and inositol 1,4,5-trisphosphate receptors in smooth muscle cells of murine feed arteries and arterioles. J. Physiol. (Lond.) 590, 1849–1869 (2012).
Barry, J.M. et al. Temporal coordination of hippocampal neurons reflects cognitive outcome post-febrile status epilepticus. EBioMedicine 7, 175–190 (2016).
Mitra, P. & Bokil, H. Observed Brain Dynamics (Oxford University Press, 2008).
Acknowledgements
The authors gratefully acknowledge J. Barry for assistance with local field potential recordings and analysis, and S. O'Dwyer and M. Ross for technical assistance. This study was supported by a postdoctoral fellowship (14POST20480144 to T.A.L.) and a scientist development grant (14SDG20150027 to M.K.) from the American Heart Association, and grants from the United Leukodystrophy Foundation (to F.D.), the Totman Medical Research Trust (to M.T.N.), Fondation Leducq (to M.T.N.), European Union's Horizon 2020 research and innovation programme (grant agreement No 666881, SVDs@target, to M.T.N.), and National Institutes of Health (R01-HL-136636 to F.D.; T32HL-007594 to A.L.G.; K01-DK103840 to N.R.T.; P30-GM-103498 to the COBRE imaging facility at UVM College of Medicine; P01-HL-095488, R01-HL-121706, R37-DK-053832, 7UM-HL-1207704 and R01-HL-131181 to M.T.N.).
Author information
Authors and Affiliations
Contributions
T.A.L. acquired and analyzed patch clamp electrophysiology data, provided tissue for RT-qPCR, acquired and analyzed in vivo hemodynamics and diameter data, acquired and analyzed local field potential data, wrote the initial draft, and edited the manuscript. F.D. developed the CaPA preparation, acquired and analyzed all CaPA preparation data and edited the manuscript. M.K. acquired and analyzed laser Doppler flowmetry data. A.L.G. acquired fluorescent-staining data and edited the manuscript. N.R.T. acquired and analyzed RT-qPCR data. J.E.B. edited the manuscript. D.H.-E. contributed significantly to the study design and edited the manuscript. M.T.N. conceived and directed the study, and wrote and edited the manuscript. All authors reviewed the manuscript and approved its submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Relative expression of KIR2.1 transcript to eNOS was reduced 29-fold in capillaries from knockout animals.
Relative expression of KIR2.1 transcript to eNOS was reduced 29-fold in capillaries from knockout animals (n = 6 capillaries from 6 mice), compared to controls (n = 5 capillaries from 5 mice) indicating successful knockout of KIR2.1 (*P = 0.0108 (t9 = 3.204); unpaired t-test). Error bars represent s.e.m.
Supplementary Figure 2 KIR currents in pial artery SM cells were unaffected by knockout of KIR2.1 in ECs.
(A) Typical experiment in a WT pial artery SM cell in which control currents (black trace) are characterized by an inwardly rectifying current at potentials negative to EK (-23 mV) and an outwardly rectifying component at positive potentials. Application of 100 μM Ba2+ inhibited the inward rectifier component (red). Currents were recorded in response to a 200-ms ramp from -140 to +50 mV with 60 mM [K+]o and 300 nM [Ca2+]i. (B) Typical experiment in a pial artery SM cell from an EC KIR2.1−/− mouse under the same conditions. Ba2+ (blue trace) inhibited the inward rectifier component that was evident under control conditions (black trace). (C) Summary data at -140 mV, indicating no difference in SM cell KIR current density between WT and EC KIR2.1−/− mice (WT, n = 14 cells from 3 mice; EC KIR2.1−/−, n = 13 cells from 4 mice; P = 0.5176 (t25 = 0.6564) Student’s unpaired t-test). (D) The density of voltage-dependent currents at +50 mV was also unchanged between SM cells from WT and EC KIR2.1−/− mice (WT, n = 14 cells from 3 mice; EC KIR2.1−/−, n = 13 cells from 4 mice; P = 0.2002 (t25 = 1.316) Student’s unpaired t-test). All error bars represent s.e.m.
Supplementary Figure 3 SK and IK channel activation in pial artery ECs.
(A) Typical experiment in a pial artery EC in which a small inwardly rectifying current (black line) is observed in response to a 200-ms ramp from -140 to +50 mV under control conditions (6 mM [K+]o, 300 nM [Ca2+]i). Subsequent application of 1 μM NS309 (green) produced large SK and IK currents. (B) Typical experiment in a pial artery EC dialyzed with 3 μM Ca2+, which caused the immediate development of prominent K+ currents.
Supplementary Figure 4 The presence of capillaries did not affect the properties of attached, pressurized (40 mmHg) parenchymal arterioles.
(A) Side-by-side comparison of vasomotor responses in parenchymal arteriole (PA) (left) and CaPA (right) preparations taken from the same mouse. Nearly identical responses were observed to bath application of 1 μM NS309, 10 mM K+, and 100 nM U46619. (B) Summary data for seven paired experiments (7 mice) indicating that PA and CaPA preparations develop the same degree of myogenic tone and have identical vasomotor properties (n = 7; tone: P = 0.7549, (t6 = 0.3268); NS309: P = 0.5995, (t6 = 0.5542); 10 mM K+: P = 0.2616, (t6 = 1,239); U46619: P = 0.7666, (t6 = 0.3107); paired Student’s t-test). All error bars represent s.e.m.
Supplementary Figure 5 K+ concentrations lower than 10 mM also dilated upstream arterioles when applied onto capillaries, with a threshold for activation between 6 and 7 mM K+ followed by moderately graded responses between 7 and 10 mM.
(A) Typical intraluminal arteriolar diameter at Zone 1, located at the point from which the primary capillary initially branches out, during stimulation of attached downstream capillaries with 6, 7, 8, 9 and 10 mM K+. (B) Summary data showing peak diameter changes in Zone 1 in response to the different K+ concentrations (n = 5 preparations from 5 mice; 6 mM K+ vs 7 mM K+: ***P = 0.0005, (t5 = 10.48); 7 mM K+ vs 8 mM K+: *P = 0.0385, (t5 = 3.038); 8 mM K+ vs 9 mM K+: *P = 0.0330, (t5 = 3.196); 9 mM K+ vs 10 mM K+: P = 0.0538, (t5 = 2,706), paired Student’s t-test). All error bars represent s.e.m.
Supplementary Figure 6 Pressure ejection of agents onto capillaries did not directly stimulate upstream arterioles.
(A) Pipette positions (tip indicated by black arrowheads) for capillary stimulation (left) and arteriole stimulation (right). (B) Pressure ejection of 1 μM NS309 onto capillaries (P1, purple) had no effect on upstream arteriolar diameter, whereas direct arteriole stimulation (P2, orange) with this agent caused substantial dilation, indicating that solutions ejected onto capillaries do not spill over onto the arteriole.
Supplementary Figure 7 Endothelial function and vasomotor properties of parenchymal arterioles were unaffected by EC KIR2.1 knockout.
(A) Typical diameter traces of pressurized parenchymal arterioles from WT (top) and EC KIR2.1−/− (bottom) mice. Myogenic tone and responses to the SK and IK channel opener NS309 were essentially identical between groups. (B) Summary of myogenic tone at 40 mm Hg intravascular pressure (top) and dilation evoked by NS309 (bottom) in WT and EC KIR2.1−/− mice (WT, n = 7 mice; EC KIR2.1−/−, n = 9 mice); tone: P = 0.2580, (t13 = 1.183); NS309: P = 0.7969, (t13 = 0.2627); unpaired Student’s t-test). All error bars represent s.e.m.
Supplementary Figure 8 Surgically removing the capillary tree from its upstream arteriole eliminated arteriolar dilation following capillary stimulation with 10 mM K+.
(A) A CaPA preparation with capillaries attached (top) or severed (middle and bottom) with a pipette positioned for capillary (top and middle) or arteriole (bottom) stimulation by pressure ejection. Red arrow indicates the tip of the pipette. Diameter was recorded in Zone 1 (black box) where the primary capillary branches from the arteriole. (B) Arteriolar diameter at Zone 1 in response to 10 mM K+ stimulation of attached capillaries (top) or severed capillaries (middle). Application of 10 mM K+ to capillaries failed to produce upstream arteriolar dilation after surgical separation of the capillary tree from the arteriole, while direct stimulation of the arteriole with 10 mM K+ still led to vasodilation (bottom). This observation confirms the spatial restriction of the pressure ejected solution and indicates that the observed phenomenon relies on inherent conducted signaling from capillaries to the arteriole. (C) Summary data (n = 6 preparations, 6 mice) showing peak diameter changes in Zone 1 in the different configurations: Capillary tree attached (top) or severed (middle and bottom), induced by 10 mM K+ applied onto capillaries (top and middle) or the arteriole (bottom). Error bars represent s.e.m.
Supplementary Figure 9 Stimulation of capillaries in vivo with 10 mM K+ increased RBC velocity in WT mice, but not in EC KIR2.1−/− mice or in the presence of Ba2+.
(A) Typical velocity-time trace for pressure ejection of 10 mM K+ (300 ms, 8 psi; purple arrow) onto a capillary in a WT mouse, showing a rapid and sustained increase in RBC velocity. Gray circles represent the velocities of individual RBCs, and the blue line is a running average. (B) Same as in A for an experiment performed on a capillary in an EC KIR2.1−/− mouse, showing the lack of a substantial increase in RBC velocity to 10 mM K+. (C, D) Typical RBC flux-time trace (C) and corresponding RBC velocity time-course (D) for pressure ejection of 10 mM K+ (200 ms, 6 psi; purple arrow) onto a WT mouse capillary in vivo after a 25-min incubation of the cortex with 100 μM Ba2+. (E) Summary data showing RBC velocity before and after capillary application of 10 mM K+ in WT mice (n = 11 paired experiments, 11 mice; ***P = 0.0004 (t10 = 5.244), paired Student’s t-test). (F) Summary data showing RBC velocity before and after capillary application of 10 mM K+ in EC KIR2.1−/− mice (n = 9 paired experiments, 9 mice; P = 0.88 (t8 = 0.1558), paired Student’s t-test). (G) Summary data showing RBC velocity before and after capillary application of 10 mM K+ in WT mice following cortical application of 100 μM Ba2+ (n = 6 paired experiments, 6 mice; P = 0.4367 (t5 = 0.8448) Student’s paired t-test). All error bars represent s.e.m.
Supplementary Figure 10 Stimulation of capillaries in vivo with 3 mM K+ had no effect on RBC flux or velocity.
(A) Typical RBC flux-time trace for pressure ejection of aCSF (3 mM K+; 300 ms, 6 psi, black arrow) onto a capillary in vivo. (B) Corresponding RBC velocity-time trace for the experiment shown in A (gray circles, individual RBC velocities; blue line, running average). (C) Summary data for RBC flux (n = 6 paired experiments, 6 mice; P = 0. 5464 (t5 = 0.6466) Student's paired t-test) and (D) velocity (n = 6 paired experiments, 6 mice; P = 0.9199 (t5 = 0.1057) Student's paired t-test) and before and after aCSF delivery, indicating that pressure ejection alone produced no change in either parameter. Error bars represent s.e.m.
Supplementary Figure 11 Capillary hyperemia to 10 mM K+ persisted in the presence of tetrodotoxin (TTX), a blocker of voltage-dependent Na+ channels.
(A) Contralateral whisker stimulation increased capillary flux under basal conditions, and this response was eliminated by application of 3 μM TTX to the cranial surface (n = 5 paired experiments, 5 mice; **P = 0.0039, two-way ANOVA with post hoc Tukey's multiple comparisons test). (B) In the presence of TTX, application of 10 mM K+ to the capillary still caused an increase in RBC flux and velocity, as evidenced by the increased number and steeper angle of RBCs (black streaks against the green FITC-loaded plasma) passing through the line-scanned region. Left: baseline distance-time line scan plot; right: after application of 10 mM K+. (C) Typical RBC flux-time plot indicating marked hyperemia after in vivo application of 10 mM K+ to a capillary, after pre-treatment with 3 μM TTX to silence neuronal activity. (D) Summary data for the peak increase in capillary RBC flux evoked by 10 mM K+ in the presence of TTX (n = 5 paired experiments, 5 mice; *P = 0.0354 (t4 = 3.123) paired Student's t-test). Error bars represent s.e.m.
Supplementary Figure 12 Whisker stimulation-evoked neural activity was unaffected by 100 μM Ba2+ superfusion.
(A) Representative 8 s LFP epoch (top) and accompanying 0-20 Hz spectrogram (bottom) recorded from the whisker barrel cortex in response to contralateral whisker stimulation under control conditions. Stimulation reliably entrained large oscillations at a frequency of ~5 Hz. (B) Exactly as in A for the same mouse, after superfusion of 100 μM Ba2+ over the cranial surface. (C) Signal spectrum plot for the recording in A illustrating the predominance of 5 Hz oscillations evoked by whisker stimulation. (D) Signal spectrum plot for the recording in B. (E) Summary data showing the peak signal amplitude before and after 100 μM Ba2+ superfusion for the 0 – 4 Hz band of the LFP spectrum (n = 5 paired experiments, 5 mice; P = 0.3778 (t4 = 0.991) Student's paired t-test). (F) Summary data showing the frequency of peak LFP oscillations before and after 100 μM Ba2+ superfusion for the 0 – 4 Hz band of the LFP spectrum (n = 5 paired experiments, 5 mice; P = 0.4804 (t4 = 0.777) Student's paired t-test). (G) Summary data showing the peak signal amplitude before and after 100 μM Ba2+ superfusion for the 4 – 6 Hz band of the LFP spectrum (n = 5 paired experiments, 5 mice; P = 0.5627 (t4 = 0.6303) Student's paired t-test). (H) Summary data showing the frequency of peak LFP oscillations before and after 100 μM Ba2+superfusion for the 4 – 6 Hz band of the LFP spectrum (n = 5 paired experiments, 5 mice; P = 0.1202 (t4 = 1.969) Student's paired t-test). (I) Summary data showing the peak signal amplitude before and after 100 μM Ba2+ superfusion for the 6 – 20 Hz band of the LFP spectrum (n = 5 paired experiments, 5 mice; P = 0.755 (t4 = 0.3337) Student's paired t-test). Note that the scale is one order of magnitude smaller than for the data in other bands, reflecting lower activity in this frequency range. (J) Summary data showing the frequency of peak LFP oscillations before and after 100 μM Ba2+superfusion for the 6 – 20 Hz band of the LFP spectrum (n = 4 paired experiments, 4 mice; P = 0.907 (t3 = 0.1233) Student's paired t-test). All error bars represent s.e.m.
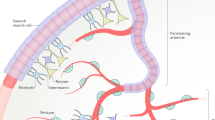
Supplementary Figure 13 Proposed mechanism for K+ regulation of CBF.
Neural activity (1) leads to an increase in local K+ around capillaries (2). Through activation of KIR channels (3), this generates local hyperpolarization of the capillary endothelial membrane, which then spreads to adjacent ECs, presumably through gap junctions, activating KIR channels to rapidly propagate a regenerative electrical signal upstream to the feed arteriole (4). After spreading into adjacent SM cells (SMC), hyperpolarization deactivates voltage-dependent Ca2+ channels (VDCC). The ensuing decrease in intracellular Ca2+ causes SM relaxation and arteriolar dilation (5), promoting an increase in blood flow into the capillaries (6).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13 and Supplementary Tables 1 and 2 (PDF 2128 kb)
Pressure ejection of 10 mM K+ onto a capillary ex vivo promotes rapid upstream arteriolar dilation.
Blue dot (top right) indicates duration of 10 mM K+ application. Note the rapid arteriolar dilation following downstream K+ delivery. (MP4 3138 kb)
Pressure ejection of 10 mM K+ onto a capillary in vivo promotes a rapid hyperemic response.
TRITC dextran (red) was included in the pipette to monitor the spatial spread of K+ delivery. The red dot (top left) indicates the very brief (200 ms) duration of 10 mM K+ application. Note the rapid increase in the flux and velocity of RBCs (visualized as dark shapes against the FITC-containing capillary lumen; green) following ejection of K+. RBC flux values are not absolute due to the rate of acquisition (1.3 Hz). Scale bar: 10 μm. (MP4 4447 kb)
Pressure ejection of 10 mM K+ onto a capillary in vivo evokes upstream arteriolar dilation.
Left: Static image showing a z-projection of the imaging field to capture capillary branches that deviate from the imaging plane of the experiment, illustrating that the stimulated capillary is downstream of the monitored arteriole. Right: Single-plane imaging experiment showing arteriolar dilation to capillary stimulation. TRITC dextran (red; included in the pipette to monitor the spatial spread of K+ delivery) fluorescence increases as K+ is ejected from the pipette onto a capillary out of the imaging plane, which leads to robust vasodilation in the upstream arteriole (white arrow). Scale bar: 10 μm. (MP4 1946 kb)
Rights and permissions
About this article
Cite this article
Longden, T., Dabertrand, F., Koide, M. et al. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci 20, 717–726 (2017). https://doi.org/10.1038/nn.4533
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4533
This article is cited by
-
Sex, hormones and cerebrovascular function: from development to disorder
Fluids and Barriers of the CNS (2024)
-
Direct association with the vascular basement membrane is a frequent feature of myelinating oligodendrocytes in the neocortex
Fluids and Barriers of the CNS (2023)
-
The CLDN5 gene at the blood-brain barrier in health and disease
Fluids and Barriers of the CNS (2023)
-
CaV3.1 channels facilitate calcium wave generation and myogenic tone development in mouse mesenteric arteries
Scientific Reports (2023)
-
Orai, RyR, and IP3R channels cooperatively regulate calcium signaling in brain mid-capillary pericytes
Communications Biology (2023)