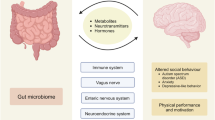
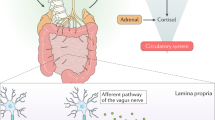
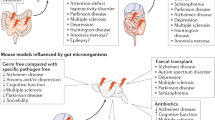
Abstract
The diverse collection of microorganisms that inhabit the gastrointestinal tract, collectively called the gut microbiota, profoundly influences many aspects of host physiology, including nutrient metabolism, resistance to infection and immune system development. Studies investigating the gut–brain axis demonstrate a critical role for the gut microbiota in orchestrating brain development and behavior, and the immune system is emerging as an important regulator of these interactions. Intestinal microbes modulate the maturation and function of tissue-resident immune cells in the CNS. Microbes also influence the activation of peripheral immune cells, which regulate responses to neuroinflammation, brain injury, autoimmunity and neurogenesis. Accordingly, both the gut microbiota and immune system are implicated in the etiopathogenesis or manifestation of neurodevelopmental, psychiatric and neurodegenerative diseases, such as autism spectrum disorder, depression and Alzheimer's disease. In this review, we discuss the role of CNS-resident and peripheral immune pathways in microbiota–gut–brain communication during health and neurological disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Debbie Maizels/Springer Nature

Debbie Maizels/Springer Nature

Debbie Maizels/Springer Nature
Similar content being viewed by others
References
Belkaid, Y. & Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 157, 121–141 (2014).
Honda, K. & Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 535, 75–84 (2016).
Rooks, M.G. & Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352 (2016).
Collins, S.M., Surette, M. & Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 10, 735–742 (2012).
Cryan, J.F. & Dinan, T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712 (2012).
Sampson, T.R. & Mazmanian, S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 17, 565–576 (2015).
Reaa, K., Dinan, T.G. & Cryan, J.F. The microbiome: a key regulator of stress and neuroinflammation. Neurobiol. Stress 4, 23–33 (2016).
Deverman, B.E. & Patterson, P.H. Cytokines and CNS development. Neuron 64, 61–78 (2009).
Stephan, A.H., Barres, B.A. & Stevens, B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu. Rev. Neurosci. 35, 369–389 (2012).
Elmer, B.M. & McAllister, A.K. Major histocompatibility complex class I proteins in brain development and plasticity. Trends Neurosci. 35, 660–670 (2012).
Weinstein, L.I., Revuelta, A. & Pando, R.H. Catecholamines and acetylcholine are key regulators of the interaction between microbes and the immune system. Ann. NY Acad. Sci. 1351, 39–51 (2015).
Baganz, N.L. & Blakely, R.D. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem. Neurosci. 4, 48–63 (2013). This reference extensively reviews the role of serotonin signaling and serotonin uptake in immune cell function, highlighting serotonergic pathways that are intrinsic to innate and adaptive immune cells.
Ahern, G.P. 5-HT and the immune system. Curr. Opin. Pharmacol. 11, 29–33 (2011).
Barragan, A., Weidner, J.M., Jin, Z., Korpi, E.R. & Birnir, B. GABAergic signalling in the immune system. Acta Physiol. (Oxf.) 213, 819–827 (2015).
Ben-Shaanan, T.L. et al. Activation of the reward system boosts innate and adaptive immunity. Nat. Med. 22, 940–944 (2016).
Erickson, M.A., Dohi, K. & Banks, W.A. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation 19, 121–130 (2012).
Banks, W.A. The blood-brain barrier in neuroimmunology: tales of separation and assimilation. Brain Behav. Immun. 44, 1–8 (2015).
Rook, G.A., Raison, C.L. & Lowry, C.A. Microbiota, immunoregulatory old friends and psychiatric disorders. Adv. Exp. Med. Biol. 817, 319–356 (2014).
Nayak, D., Roth, T.L. & McGavern, D.B. Microglia development and function. Annu. Rev. Immunol. 32, 367–402 (2014).
Matcovitch-Natan, O. et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 353, aad8670 (2016).
Nayak, D., Zinselmeyer, B.H., Corps, K.N. & McGavern, D.B. In vivo dynamics of innate immune sentinels in the CNS. Intravital 1, 95–106 (2012).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
Bilbo, S.D. & Schwarz, J.M. The immune system and developmental programming of brain and behavior. Front. Neuroendocrinol. 33, 267–286 (2012).
Hu, X. et al. Microglial and macrophage polarization—new prospects for brain repair. Nat. Rev. Neurol. 11, 56–64 (2015).
Ransohoff, R.M. A polarizing question: do M1 and M2 microglia exist? Nat. Neurosci. 19, 987–991 (2016).
Bogie, J.F.J., Stinissen, P. & Hendriks, J.J.A. Macrophage subsets and microglia in multiple sclerosis. Acta Neuropathol. 128, 191–213 (2014).
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015). This study demonstrates the critical role of the microbiome in modulating microglial development and maintenance, particularly how short-chain fatty acids promote microglial maturity.
Borre, Y.E. et al. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol. Med. 20, 509–518 (2014).
Smith, P.M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Schafer, D.P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Cho, I. et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621–626 (2012).
Khakh, B.S. & Sofroniew, M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 18, 942–952 (2015).
Jensen, C.J., Massie, A. & De Keyser, J. Immune players in the CNS: the astrocyte. J. Neuroimmune Pharmacol. 8, 824–839 (2013).
Rossi, D. Astrocyte physiopathology: at the crossroads of intercellular networking, inflammation and cell death. Prog. Neurobiol. 130, 86–120 (2015).
Barres, B.A. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60, 430–440 (2008).
Dong, Y. & Benveniste, E.N. Immune function of astrocytes. Glia 36, 180–190 (2001).
Rothhammer, V. et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 22, 586–597 (2016). This study highlights how microbial metabolites of dietary tryptophan affect astrocytic inflammatory status, which modulates the severity of EAE.
Zelante, T. et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385 (2013).
Wikoff, W.R. et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 106, 3698–3703 (2009).
Radjavi, A., Smirnov, I., Derecki, N. & Kipnis, J. Dynamics of the meningeal CD4+ T-cell repertoire are defined by the cervical lymph nodes and facilitate cognitive task performance in mice. Mol. Psychiatry 19, 531–533 (2014).
Ribatti, D. The crucial role of mast cells in blood-brain barrier alterations. Exp. Cell Res. 338, 119–125 (2015).
Forsythe, P. Microbes taming mast cells: implications for allergic inflammation and beyond. Eur. J. Pharmacol. 778, 169–175 (2016).
Khosravi, A. et al. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 15, 374–381 (2014).
Lee, Y.K., Menezes, J.S., Umesaki, Y. & Mazmanian, S.K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 108 (Suppl. 1), 4615–4622 (2011).
Ochoa-Reparaz, J. et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 183, 6041–6050 (2009).
Gaboriau-Routhiau, V. et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689 (2009).
Ivanov, I.I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Komiyama, Y., Nakae, S., Matsuki, T., Nambu, A., Ishigame, H. & Kakuta, S. et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177, 566–573 (2006).
Round, J.L. et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332, 974–977 (2011).
Round, J.L. & Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 107, 12204–12209 (2010).
Ochoa-Reparaz, J. et al. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J. Immunol. 185, 4101–4108 (2010).
Ochoa-Repáraz, J. et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 3, 487–495 (2010).
Wang, Y. et al. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nat. Commun. 5, 4432 (2014)Refs. 51–53 describe roles for Bacteroides fragilis derived PSA in neuroprotection from EAE through induction of regulatory T cell responses, highlighting the relationship between bacterially derived molecules, immune regulation and neuroinflammation.
Haghikia, A. et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43, 817–829 (2015).
Kadowaki, A. et al. Gut environment-induced intraepithelial autoreactive CD4+ T cells suppress central nervous system autoimmunity via LAG-3. Nat. Commun. 7, 11639 (2016).
Benakis, C. et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 22, 516–523 (2016).
Winek, K. et al. Depletion of cultivatable gut microbiota by broad-spectrum antibiotic pretreatment worsens outcome after murine stroke. Stroke 47, 1354–1363 (2016).
Singh, V. et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J. Neurosci. 36, 7428–7440 (2016). Refs. 56–58 describe a role for the gut microbiota in the development of brain injury in the MCAO mouse model of stroke. MCAO results in intestinal dysbiosis, which regulates disease outcome through modulation of the adaptive immune system.
Houlden, A. et al. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav. Immun. 57, 10–20 (2016).
Kigerl, K.A. et al. Gut dysbiosis impairs recovery after spinal cord injury. J. Exp. Med. 213, 2603–2620 (2016).
Walsh, J.T. et al. MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J. Clin. Invest. 125, 2547 (2015).
Gadani, S.P., Walsh, J.T., Smirnov, I., Zheng, J. & Kipnis, J. The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron 85, 703–709 (2015).
Matsushita, T., Yanaba, K., Bouaziz, J.D., Fujimoto, M. & Tedder, T.F. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J. Clin. Invest. 118, 3420–3430 (2008).
Pöllinger, B. et al. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J. Exp. Med. 206, 1303–1316 (2009).
Yaddanapudi, K. et al. Passive transfer of streptococcus-induced antibodies reproduces behavioral disturbances in a mouse model of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Mol. Psychiatry 15, 712–726 (2010).
Tennoune, N. et al. Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide α-MSH, at the origin of eating disorders. Transl. Psychiatry 4, e458 (2014).
D'Mello, C. & Swain, M.G. Liver-brain interactions in inflammatory liver diseases: implications for fatigue and mood disorders. Brain Behav. Immun. 35, 9–20 (2014).
Graff, L.A., Walker, J.R. & Bernstein, C.N. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm. Bowel Dis. 15, 1105–1118 (2009).
D'Mello, C. et al. Probiotics improve inflammation-associated sickness behavior by altering communication between the peripheral immune system and the brain. J. Neurosci. 35, 10821–10830 (2015).
Humann, J. et al. Bacterial peptidoglycan transverses the placenta to induce fetal neuroproliferation and aberrant postnatal behavior. Cell Host Microbe 19, 388–399 (2016).
Ogbonnaya, E.S. et al. Adult hippocampal neurogenesis is regulated by the microbiome. Biol. Psychiatry 78, e7–e9 (2015).
Möhle, L. et al. Ly6Chi monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. 15, 1945–1956 (2016). This study demonstrates that the gut microbiota promote hippocampal neurogenesis in adult mice through recruitment of monocytes to the CNS. However, ref. 74 observed that the gut microbiota inhibit this process, suggesting complex interactions between intestinal microbes and neurogenesis.
Hsiao, E.Y. et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463 (2013).
Buffington, S.A. et al. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 165, 1762–1775 (2016).
Choi, G.B. et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939 (2016). This study demonstrates an important role for T cell–derived IL-17A in the development of behavioral abnormalities in the maternal immune activation mouse model of ASD, highlighting the relationship between immune dysregulation, neurophysiology and behavior.
Hsiao, E.Y., McBride, S.W., Chow, J., Mazmanian, S.K. & Patterson, P.H. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc. Natl. Acad. Sci. USA 109, 12776–12781 (2012).
Smith, S.E., Li, J., Garbett, K., Mirnics, K. & Patterson, P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 27, 10695–10702 (2007).
Krishnan, V. & Nestler, E.J. The molecular neurobiology of depression. Nature 455, 894–902 (2008).
Miller, A.H. & Raison, C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 16, 22–34 (2016).
Diaz Heijtz, R. et al. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 108, 3047–3052 (2011).
Bercik, P. et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 23, 1132–1139 (2011).
Desbonnet, L. et al. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 170, 1179–1188 (2010).
Arseneault-Bréard, J. et al. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br. J. Nutr. 107, 1793–1799 (2012).
Bravo, J.A. et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 108, 16050–16055 (2011).
De Palma, G. et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat. Commun. 6, 7735 (2015).
Zheng, P. et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol. Psychiatry 21, 786–796 (2016).
Kelly, J.R. et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 82, 109–118 (2016).
Desbonnet, L., Garrett, L., Clarke, G., Bienenstock, J. & Dinan, T.G. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 43, 164–174 (2008).
Bellavance, M.A. & Rivest, S. The HPA – immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front. Immunol. 5, 136 (2014). This study discusses the immunomodulatory effects of glucocorticoids released by activation of the HPA axis, a neuroendocrine pathway that allows host adaptation to physical and psychological stress.
Hammond, C.J. et al. Immunohistological detection of Chlamydia pneumoniae in the Alzheimer's disease brain. BMC Neurosci. 11, 121 (2010).
Huang, W.S. et al. Association between Helicobacter pylori infection and dementia. J. Clin. Neurosci. 21, 1355–1358 (2014).
Karim, S. et al. An association of virus infection with type 2 diabetes and Alzheimer's disease. CNS Neurol. Disord. Drug Targets 13, 429–439 (2014).
Lurain, N.S. et al. Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J. Infect. Dis. 208, 564–572 (2013).
Pisa, D., Alonso, R., Rábano, A., Rodal, I. & Carrasco, L. Different brain regions are infected with fungi in Alzheimer's disease. Sci. Rep. 5, 15015 (2015).
Prandota, J. Possible link between Toxoplasma gondii and the anosmia associated with neurodegenerative diseases. Am. J. Alzheimers Dis. Other Demen. 29, 205–214 (2014).
Glass, C.K., Saijo, K., Winner, B., Marchetto, M.C. & Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934 (2010).
Kumar, D.K. et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci. Transl. Med. 8, 340ra72 (2016). This study demonstrates that bacterial infection promotes amyloid-β peptide aggregation as an antimicrobial response, raising the question of whether neurodegeneration in Alzheimer's disease is causally associated with host responses to microbial infection.
Harach, T. et al. Reduction of Alzheimer's disease beta-amyloid pathology in the absence of gut microbiota. Preprint at https://arxiv.org/abs/1509.02273 (2015).
Minter, M.R. et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer's disease. Sci. Rep. 6, 30028 (2016).
Chapman, M.R. et al. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295, 851–855 (2002).
Fröhlich, E.E. et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 56, 140–155 (2016).
Wang, T. et al. Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Benef. Microbes 6, 707–717 (2015).
Fasano, A., Visanji, N.P., Liu, L.W., Lang, A.E. & Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson's disease. Lancet Neurol. 14, 625–639 (2015).
Shannon, K.M. et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson's disease. Mov. Disord. 27, 709–715 (2012).
Scheperjans, F. et al. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov. Disord. 30, 350–358 (2015).
Keshavarzian, A. et al. Colonic bacterial composition in Parkinson's disease. Mov. Disord. 30, 1351–1360 (2015).
Devos, D. et al. Colonic inflammation in Parkinson's disease. Neurobiol. Dis. 50, 42–48 (2013).
Forsyth, C.B. et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson's disease. PLoS One 6, e28032 (2011).
Chen, S.G. et al. Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged Fischer 344 rats and Caenorhabditis elegans. Sci. Rep. 6, 34477 (2016).
Crack, P.J. & Bray, P.J. Toll-like receptors in the brain and their potential roles in neuropathology. Immunol. Cell Biol. 85, 476–480 (2007).
Brenchley, J.M. & Douek, D.C. Microbial translocation across the GI tract. Annu. Rev. Immunol. 30, 149–173 (2012).
Chakravarty, S. & Herkenham, M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J. Neurosci. 25, 1788–1796 (2005).
Qin, L. et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55, 453–462 (2007).
Arentsen, T. et al. The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol. Psychiatry http://dx.doi.org/10.1038/mp.2016.182 (2016).
Koh, A., De Vadder, F., Kovatcheva-Datchary, P. & Bäckhed, F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345 (2016).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015). This study identifies a network of lymphatic vessels in the meningeal spaces of the CNS, challenging the idea that the brain lacks an organized immune surveillance system.
Reigstad, C.S. et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 29, 1395–1403 (2015).
Yano, J.M. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015).
Lyte, M. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 9, e1003726 (2013).
Gershon, M.D. & Tack, J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132, 397–414 (2007).
Asano, Y. et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G1288–G1295 (2012).
Williams, B.B. et al. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe 16, 495–503 (2014).
Borovikova, L.V. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000).
van der Kleij, H., O'Mahony, C., Shanahan, F., O'Mahony, L. & Bienenstock, J. Protective effects of Lactobacillus rhamnosus and Bifidobacterium infantis in murine models for colitis do not involve the vagus nerve. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R1131–R1137 (2008).
Ait-Belgnaoui, A. et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 37, 1885–1895 (2012).
Demaude, J., Salvador-Cartier, C., Fioramonti, J., Ferrier, L. & Bueno, L. Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: implications for delayed epithelial barrier dysfunction. Gut 55, 655–661 (2006).
Moussaoui, N. et al. Changes in intestinal glucocorticoid sensitivity in early life shape the risk of epithelial barrier defect in maternal-deprived rats. PLoS One 9, e88382 (2014).
Lennon, E.M. et al. Early life stress triggers persistent colonic barrier dysfunction and exacerbates colitis in adult IL-10−/− mice. Inflamm. Bowel Dis. 19, 712–719 (2013).
Gue, M., Junien, J.L. & Bueno, L. Conditioned emotional response in rats enhances colonic motility through the central release of corticotropin-releasing factor. Gastroenterology 100, 964–970 (1991).
Gué, M., Peeters, T., Depoortere, I., Vantrappen, G. & Buéno, L. Stress-induced changes in gastric emptying, postprandial motility, and plasma gut hormone levels in dogs. Gastroenterology 97, 1101–1107 (1989).
Rubio, C.A. & Huang, C.B. Quantification of the sulphomucin-producing cell population of the colonic mucosa during protracted stress in rats. In Vivo 6, 81–84 (1992).
Da Silva, S. et al. Stress disrupts intestinal mucus barrier in rats via mucin O-glycosylation shift: prevention by a probiotic treatment. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G420–G429 (2014).
Park, A.J. et al. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol. Motil. 25, 733–e575 (2013).
Hueston, C.M. & Deak, T. The inflamed axis: the interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic-pituitary-adrenal axis. Physiol. Behav. 124, 77–91 (2014).
Jangi, S. et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 7, 12015 (2016).
Tremlett, H. et al. Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur. J. Neurol. 23, 1308–1321 (2016).
Chen, J. et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 6, 28484 (2016).
Miyake, S. et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS One 10, e0137429 (2015).
Cantarel, B.L. et al. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J. Investig. Med. 63, 729–734 (2015).
Keshavarzian, A. et al. Colonic bacterial composition in Parkinson's disease. Mov. Disord. 30, 1351–1360 (2015).
Bu, X.L. et al. A study on the association between infectious burden and Alzheimer's disease. Eur. J. Neurol. 22, 1519–1525 (2015).
Gungor, B., Adiguzel, E., Gursel, I., Yilmaz, B. & Gursel, M. Intestinal microbiota in patients with spinal cord injury. PLoS One 11, e0145878 (2016).
Fouts, D.E. et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J. Transl. Med. 10, 174 (2012).
Aizawa, E. et al. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 202, 254–257 (2016).
Jiang, H. et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 48, 186–194 (2015).
Messaoudi, M. et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 105, 755–764 (2011).
Mohammadi, A.A. et al. The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: a randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosci. 19, 387–395 (2016).
Tomova, A. et al. Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 138, 179–187 (2015).
Wang, L. et al. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism 4, 42 (2013).
De Angelis, M. et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One 8, e76993 (2013).
Kang, D.-W. et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One 8, e68322 (2013).
Williams, B.L. et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One 6, e24585 (2011).
Adams, J.B., Johansen, L.J., Powell, L.D., Quig, D. & Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 11, 22 (2011).
Finegold, S.M. et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 16, 444–453 (2010).
Parracho, H.M.R.T., Bingham, M.O., Gibson, G.R. & McCartney, A.L. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 54, 987–991 (2005).
Acknowledgements
The authors are supported by funding from the NIH Director's Early Independence award (5DP5OD017924 to E.Y.H.), Alfred P. Sloan Fellowship in Neuroscience (to E.Y.H.), NIH Ruth L. Kirschstein National Research Service Award (T32GM065823 to C.A.O.) and UCLA Life Sciences Division, Department of Integrative Biology & Physiology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Fung, T., Olson, C. & Hsiao, E. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci 20, 145–155 (2017). https://doi.org/10.1038/nn.4476
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4476
This article is cited by
-
The influence of placenta microbiota of normal term pregnant women on immune regulation during pregnancy
BMC Pregnancy and Childbirth (2024)
-
Acute neutrophilic vasculitis (leukocytoclasia) in 36 COVID-19 autopsy brains
Diagnostic Pathology (2024)
-
Vitamin B12 produced by gut bacteria modulates cholinergic signalling
Nature Cell Biology (2024)
-
Gut microbiota-induced CXCL1 elevation triggers early neuroinflammation in the substantia nigra of Parkinsonian mice
Acta Pharmacologica Sinica (2024)
-
The functional roles of short chain fatty acids as postbiotics in human gut: future perspectives
Food Science and Biotechnology (2024)