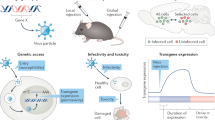
Abstract
A fundamental impediment to understanding the brain is the availability of inexpensive and robust methods for targeting and manipulating specific neuronal populations. The need to overcome this barrier is pressing because there are considerable anatomical, physiological, cognitive and behavioral differences between mice and higher mammalian species in which it is difficult to specifically target and manipulate genetically defined functional cell types. In particular, it is unclear the degree to which insights from mouse models can shed light on the neural mechanisms that mediate cognitive functions in higher species, including humans. Here we describe a novel recombinant adeno-associated virus that restricts gene expression to GABAergic interneurons within the telencephalon. We demonstrate that the viral expression is specific and robust, allowing for morphological visualization, activity monitoring and functional manipulation of interneurons in both mice and non-genetically tractable species, thus opening the possibility to study GABAergic function in virtually any vertebrate species.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
29 November 2016
In the version of this article initially published, authors Joshua S. Grimley, Anne-Rachel Krostag and Ajamete Kaykas were missing. These authors have been inserted into the author list after Jianhua Chu; they are at the Allen Institute for Brain Science, Seattle, Washington, USA, and performed experiments related to hESCs. The error has been corrected in the HTML and PDF versions of the article.
29 May 2017
The authors wish to acknowledge a potentially relevant work that they were made aware of after publication: Lee, A.T., Vogt, D., Rubenstein, J.L. & Sohal, V.S. A class of GABAergic neurons in the prefrontal cortex sends long-range projections to the nucleus accumbens and elicits acute avoidance behavior. J. Neurosci. 34, 11519–11525, http://dx.doi.org/10.1523/JNEUROSCI.1157-14.2014 (2014). This work describes an approach for using Dlx1/2 enhancers to attempt to achieve selective expression in cortical GABAergic interneurons. Although thorough validation was not performed, the results are consistent with the possibility that other regulatory elements may be generalizable as an effective way of targeting specific cell types.
01 July 2017
Nat. Neurosci. 19, 1743–1749 (2016); published online 31 October 2016; corrected after print 29 November 2016 In the version of this article initially published, authors Joshua S. Grimley, Anne-Rachel Krostag and Ajamete Kaykas were missing. These authors have been inserted into the author list after Jianhua Chu; they are at the Allen Institute for Brain Science, Seattle, Washington, USA, and performed experiments related to hESCs.
References
Rudy, B., Fishell, G., Lee, S. & Hjerling-Leffler, J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 71, 45–61 (2011).
Marín, O. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci. 13, 107–120 (2012).
Kepecs, A. & Fishell, G. Interneuron cell types are fit to function. Nature 505, 318–326 (2014).
Taniguchi, H. et al. A resource of Cre driver lines for genetic targeting of gabaergic neurons in cerebral cortex. Neuron 71, 995–1013 (2011).
Nathanson, J.L., Yanagawa, Y., Obata, K. & Callaway, E.M. Preferential labeling of inhibitory and excitatory cortical neurons by endogenous tropism of adeno-associated virus and lentivirus vectors. Neuroscience 161, 441–450 (2009).
Han, X. et al. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron 62, 191–198 (2009).
Nassi, J.J., Avery, M.C., Cetin, A.H., Roe, A.W. & Reynolds, J.H. Optogenetic activation of normalization in alert macaque visual cortex. Neuron 86, 1504–1517 (2015).
Kotterman, M.A. & Schaffer, D.V. Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 15, 445–451 (2014).
Delzor, A. et al. Restricted transgene expression in the brain with cell-type specific neuronal promoters. Hum. Gene Ther. Methods 23, 242–254 (2012).
Chen, Y.J. et al. Use of “MGE enhancers” for labeling and selection of embryonic stem cell-derived medial ganglionic eminence (MGE) progenitors and neurons. PLoS One 8, e61956 (2013).
Marik, S.A., Yamahachi, H., McManus, J.N., Szabo, G. & Gilbert, C.D. Axonal dynamics of excitatory and inhibitory neurons in somatosensory cortex. PLoS Biol. 8, e1000395 (2010).
Nathanson, J.L., Yanagawa, Y., Obata, K. & Callaway, E.M. Preferential labeling of inhibitory and excitatory cortical neurons by endogenous tropism of adeno-associated virus and lentivirus vectors. Neuroscience 161, 441–450 (2009).
Dittgen, T. et al. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc. Natl. Acad. Sci. USA 101, 18206–18211 (2004).
Kügler, S., Kilic, E. & Bähr, M. Human synapsin 1 gene promoter confers highly neuron-specific long-term transgene expression from an adenoviral vector in the adult rat brain depending on the transduced area. Gene Ther. 10, 337–347 (2003).
Wu, Z., Yang, H. & Colosi, P. Effect of genome size on AAV vector packaging. Mol. Ther. 18, 80–86 (2010).
Choi, J.-H. et al. Optimization of AAV expression cassettes to improve packaging capacity and transgene expression in neurons. Mol. Brain 7, 17 (2014).
Zerucha, T. et al. A highly conserved enhancer in the Dlx5/Dlx6 intergenic region is the site of cross-regulatory interactions between Dlx genes in the embryonic forebrain. J. Neurosci. 20, 709–721 (2000).
Ghanem, N., Yu, M., Poitras, L., Rubenstein, J.L.R. & Ekker, M. Characterization of a distinct subpopulation of striatal projection neurons expressing the Dlx genes in the basal ganglia through the activity of the I56ii enhancer. Dev. Biol. 322, 415–424 (2008).
Visel, A. et al. A high-resolution enhancer atlas of the developing telencephalon. Cell 152, 895–908 (2013).
Ghanem, N. et al. Regulatory roles of conserved intergenic domains in vertebrate Dlx bigene clusters. Genome Res. 13, 533–543 (2003).
Stühmer, T., Puelles, L., Ekker, M. & Rubenstein, J.L.R. Expression from a Dlx gene enhancer marks adult mouse cortical GABAergic neurons. Cereb. Cortex 12, 75–85 (2002).
Stenman, J., Toresson, H. & Campbell, K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J. Neurosci. 23, 167–174 (2003).
Monory, K. et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 51, 455–466 (2006).
Miyoshi, G. et al. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J. Neurosci. 30, 1582–1594 (2010).
Cobos, I., Long, J.E., Thwin, M.T. & Rubenstein, J.L. Cellular patterns of transcription factor expression in developing cortical interneurons. Cereb. Cortex 16 (Suppl. 1), i82–i88 (2006).
Xu, X., Roby, K.D. & Callaway, E.M. Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J. Comp. Neurol. 499, 144–160 (2006).
Punnakkal, P., von Schoultz, C., Haenraets, K., Wildner, H. & Zeilhofer, H.U. Morphological, biophysical and synaptic properties of glutamatergic neurons of the mouse spinal dorsal horn. J. Physiol. (Lond.) 592, 759–776 (2014).
Fenno, L.E. et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nat. Methods 11, 763–772 (2014).
Roth, B.L. DREADDs for Neuroscientists. Neuron 89, 683–694 (2016).
Armbruster, B.N., Li, X., Pausch, M.H., Herlitze, S. & Roth, B.L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. USA 104, 5163–5168 (2007).
Alexander, G.M. et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63, 27–39 (2009).
Zou, D. et al. DREADD in parvalbumin interneurons of the dentate gyrus modulates anxiety, social interaction and memory extinction. Curr. Mol. Med. 16, 91–102 (2016).
Urban, D.J. & Roth, B.L. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu. Rev. Pharmacol. Toxicol. 55, 399–417 (2015).
Nord, A.S., Pattabiraman, K., Visel, A. & Rubenstein, J.L.R. Genomic perspectives of transcriptional regulation in forebrain development. Neuron 85, 27–47 (2015).
Krook-Magnuson, E., Armstrong, C., Oijala, M. & Soltesz, I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat. Commun. 4, 1376 (2013).
Kätzel, D., Nicholson, E., Schorge, S., Walker, M.C. & Kullmann, D.M. Chemical-genetic attenuation of focal neocortical seizures. Nat. Commun. 5, 3847 (2014).
Whalen, S., Truty, R.M. & Pollard, K.S. Enhancer-promoter interactions are encoded by complex genomic signatures on looping chromatin. Nat. Genet. 48, 488–496 (2016).
Guerrier, S. et al. The F-BAR domain of srGAP2 induces membrane protrusions required for neuronal migration and morphogenesis. Cell 138, 990–1004 (2009).
Saunders, A., Johnson, C.A. & Sabatini, B.L. Novel recombinant adeno-associated viruses for Cre activated and inactivated transgene expression in neurons. Front. Neural Circuits 6, 47 (2012).
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Mattis, J. et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat. Methods 9, 159–172 (2011).
Krashes, M.J. et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011).
Lu, C. et al. Micro-electrode array recordings reveal reductions in both excitation and inhibition in cultured cortical neuron networks lacking Shank3. Mol. Psychiatry 21, 159–168 (2016).
Paull, D. et al. Automated, high-throughput derivation, characterization and differentiation of induced pluripotent stem cells. Nat. Methods 12, 885–892 (2015).
Shi, Y., Kirwan, P. & Livesey, F.J. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat. Protoc. 7, 1836–1846 (2012).
Maroof, A.M. et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell 12, 559–572 (2013).
Vallentin, D., Kosche, G., Lipkind, D. & Long, M.A. Neural circuits. Inhibition protects acquired song segments during vocal learning in zebra finches. Science 351, 267–271 (2016).
Kotak, V.C. et al. Hearing loss raises excitability in the auditory cortex. J. Neurosci. 25, 3908–3918 (2005).
Chen, Q. et al. Imaging neural activity using Thy1-GCaMP transgenic mice. Neuron 76, 297–308 (2012).
Acknowledgements
We thank S. Gerard, L. Sjulson and T. Petros for discussions and comments on the manuscript. Plasmids AAV-CaMKIIa-GCaMP6f-P2A-nls-dTomato and AAV-hSyn1-GCaMP6s-P2A-nls-dTomato were a gift from J. Ting (Allen Institute for Brain Science). Plasmid pNeuroD-Ires-GFP was a gift from F. Polleux (Department of Neuroscience, Mortimer B. Zuckerman Mind Brain Behavior Institute, Columbia University Medical Center). Plasmid pGP-CMV-GCaMP6f was a gift from D. Kim (Janelia Research Campus, Howard Hughes Medical Institute). Plasmid pAAV-Ef1a-DIO hChR2(E123T/T159C)-EYFP was a gift from K. Deisseroth (Department of Bioengineering, Stanford University). Plasmid pAAV-hSyn-DIO-hM3D(Gq)-mCherry was a gift from B. Roth (Department of Pharmacology, UNC Chapel Hill Medical School).This work was supported by grants from the National Institutes of Health (NIH): MH071679, NS08297, NS074972, MH095147, as well as support from the Simons Foundation (SFARI) (to G.F.); MH066912 (to S.S.A.); and EY022577 and MH063912 (to E.M.C.).
Author information
Authors and Affiliations
Contributions
J.D. conceived and designed the viral constructs, designed and performed experiments, analyzed data, prepared figures and wrote the manuscript. Q.C., C.L. and R.L. performed experiments, analyzed data and prepared figures related to mouse primary cultures. R.T. performed the slice recording experiments, analyzed the data and prepared the associated figures and text. G.-A.S. and S.L.R. performed viral injections and IHC on mice. Q.X. and L.G. produced the viruses at NYUAD using plasmids conceived and generated at NYU. G.K. performed experiments related to zebra finches. A.L.J., G.B.S. and D.E.W. performed experiments related to ferrets. V.C.K. and T.M.M. performed experiments related to gerbils. J.H.R., M.C.A. and M.S.R. performed experiments related to marmosets. I.K. and T.R. performed experiments related to iPSCs. J.C., S.A., J.S.G., A.-R.K. and A.K. performed experiments related to hESCs, M.B. and J.S.D. performed experiments related to spinal cord. S.A.A., E.M.C., D.J.S., D.F., V.F., M.A.L., S.N., J.H.R., D.H.S., B.R. and G. Feng helped with the study design. G. Fishell helped with study design, manuscript and figure preparation and supervised the project. All authors edited and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The New York University Langone Medical Center has filed patent applications related to this work with J.D and G.F. listed as inventors.
Integrated supplementary information
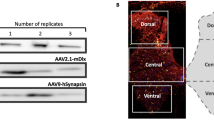
Supplementary Figure 1 rAAV-mDlx-GCaMP6f allows calcium transients to be visualized in interneurons in vitro
(a) The indicated rAAV was added to primary cortical culture at DIV8 and analyzed at DIV19 by immunostaining using GFP and GAD67 antibodies and counterstained with Dapi. Scale bars represent 20μm. (b) Visualization of ΔF/F calcium current traces recorded from GCaMP6f-expressing cortical interneurons. Each trace corresponds to a single neuron.
Supplementary Figure 2 rAAV-mDlx-GFP is not selective for interneurons in the spinal cord
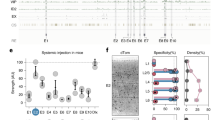
(a, b) P0 wildtype Swiss Webster mice (n=10) were injected with the indicated virus in the lumbar spinal cord and analyzed by immunostaining for GFP and PAX2 immunoreactivity after one weeks. Representative example of expression of the reporter GFP and Pax2 and corresponding quantifications. (Upper panel: 2.9 +/- 1.7%, n= 255 cells from 4 animals; lower panel: 12.2 +/- 1.1%, n= 1904 cells from 6 animals). Quantification are indicated in the text as mean ± s.e.m and are represented as box-and-whisker plot with upper and lower whiskers represent the maximum and minimum value respectively and the box represent upper, median and lower quartile. Dashed lines represent limits of the spinal cord. Scale bars represent 25μm (left merge) 100μm (right merge).
Supplementary Figure 3 rAAV-mDlx-Flex-GFP allows intersectional targeting of Cck-expressing interneurons
(a) Adult CCK-Cre mice (n=4) or wildtype C57Bl6 mice (n=2) were injected with the indicated virus in the somatosensory cortex (S1) and were analyzed for GFP expression. (b) Adult wildtype C57Bl6 mice (n=2) were injected bilaterally with rAAV-mDlx-Flex-GFP and analyzed by immunostaining for GAD67 immunoreactivity after 2 weeks. Note that no cells expressing could be observed (n=4 injection sites from 2 animals). Scale bars represent 100μm (a) or 10 μm (b).
Supplementary Figure 4 rAAV-hDlx-Gq-DREADD restricts reporter expression to the membrane of GABAergic interneurons
(a) Scheme of the rAAV-hDlx-Gq viral construct. (a-c) Adult Dlx6aCre::RCE (n=4) or (d) C57Bl6 mice (n=16) were injected with rAAV-hDlx-Flex-GFP in somatosensory cortex (S1) or hippocampus (CA1) and were analyzed by immunostaining for the indicated markers after 7 days. Representative example of expression of the reporter dTomato and the indicated marker and corresponding quantifications (S1: 91.9 +/- 0.7%, n= 2091 cells from 4 animals; CA1: 93.9 +/- 2.3%, n= 173 cells from 3 animals). Quantification are indicated in the text as mean ± s.e.m and are represented as box-and-whisker plot with upper and lower whiskers represent the maximum and minimum value respectively and the box represent upper, median and lower quartile. Dashed lines represent limits of the indicated anatomical structures. P – pyramidal layer. Scale bars represent 100μm (a), 25μm (c,d).
Supplementary Figure 5 rAAV-mDlx-GFP allows in vivo recording of interneurons in the HVC of zebra finches
Adult Zebra Finches (n=4) were injected with rAAV-mDlx-GFP in HVC and were either sectioned for electrophysiological recording after 2 weeks or a cranial window was placed above the injection site for two-photon imaging and in-vivo electrophysiological recording. (a, b) Representative examples of biocytin GFP positive cells recorded from acute slice showing typical neocortical interneuron morphology and corresponding membrane potential of cells in response to current pulses. (c) Representative examples of biocytin GFP positive recorded in vivo and membrane potential of the two cells marked with stars in response to current pulses. Dark gray: hyperpolarizing; Black trace: rheobase; light gray: -200pA / twice rheobase. Scale bars represent 20μm (a, b) or 10μm (c).
Supplementary Figure 6 rAAV-mDlx-ChR2-mCherry allows modulation of neuronal activity in gerbil auditory cortex
Adult gerbils were stereotactically injected with 50-100nl of rAAV-mDlx-ChR2-mCherry in auditory cortex (A1) and sectioned for optogenetics and electrophysiological recording after 5 weeks (n=6 animals). (a) Infrared and fluorescence images showing the cells targeted for electrophysiological recording. Dashed lines highlight the edges of the recording pipet. Note that the cell in (c) does not express mCherry but is surrounded by mCherry-expressing cells. (b) Left panel: Membrane potential of a mCherry+ cell in response current pulses corresponding to twice the rheobase. Baseline membrane potential is -60 to -70 mV. Right panel: intensity-dependent increase of firing upon 1ms pulse of blue light for both FS and LTS interneurons. (c) Left panel: Membrane potential of a cell mCherry- in response current pulses corresponding to twice the rheobase. Baseline membrane potential is -60 to -70 mV. Right panel: the firing of pyramidal neurons is interrupted by a 1ms pulse of blue light suggesting activation of interneurons produces such inhibitory postsynaptic response. Scale bars represent 5μm.
Supplementary Figure 7 rAAV-mDlx-GCaMP6f allows the visualization of calcium transients in interneurons in ferret visual cortex
Ferrets aged P27-P30 were injected with rAAV-mDlx-GCaMP6f in primary visual cortex and then imaged at P50 (n=4 animals). (a) rAAV-mDlx-GCaMP6f labeled large numbers of inhibitory neurons, allowing for in vivo two-photon calcium imaging of inhibitory neurons. (b) Representative recording of calcium fluorescence of individual neurons upon presentation of visual stimulus. Labeled neurons show robust calcium fluorescence changes to visual stimuli during functional imaging. Black traces represent mean over 8 trials and grey represent ± s.e.m. Blue bars above responses indicate period of stimulus presentation. PS: preferred stimulus. NPS: non preferred stimulus. Scale bar represents 100 μm.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 1179 kb)
Rights and permissions
About this article
Cite this article
Dimidschstein, J., Chen, Q., Tremblay, R. et al. A viral strategy for targeting and manipulating interneurons across vertebrate species. Nat Neurosci 19, 1743–1749 (2016). https://doi.org/10.1038/nn.4430
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4430
This article is cited by
-
An optrode array for spatiotemporally-precise large-scale optogenetic stimulation of deep cortical layers in non-human primates
Communications Biology (2024)
-
Synaptic wiring motifs in posterior parietal cortex support decision-making
Nature (2024)
-
Central medial thalamic nucleus dynamically participates in acute itch sensation and chronic itch-induced anxiety-like behavior in male mice
Nature Communications (2023)
-
A cell-type-specific error-correction signal in the posterior parietal cortex
Nature (2023)
-
Anatomical and molecular characterization of parvalbumin-cholecystokinin co-expressing inhibitory interneurons: implications for neuropsychiatric conditions
Molecular Psychiatry (2023)