Abstract
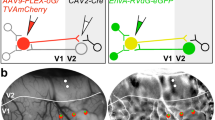
Neurons in the thalamorecipient layers of sensory cortices integrate thalamic and recurrent cortical input. Cortical neurons form fine-scale, functionally cotuned networks, but whether interconnected cortical neurons within a column process common thalamocortical inputs is unknown. We tested how local and thalamocortical connectivity relate to each other by analyzing cofluctuations of evoked responses in cortical neurons after photostimulation of thalamocortical axons. We found that connected pairs of pyramidal neurons in layer (L) 4 of mouse visual cortex share more inputs from the dorsal lateral geniculate nucleus than nonconnected pairs. Vertically aligned connected pairs of L4 and L2/3 neurons were also preferentially contacted by the same thalamocortical axons. Our results provide a circuit mechanism for the observed amplification of sensory responses by L4 circuits. They also show that sensory information is concurrently processed in L4 and L2/3 by columnar networks of interconnected neurons contacted by the same thalamocortical axons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
12 July 2016
In the version of this article initially published online, the abstract referred to connected pairs of L4 and L2 and 3 (L2/3) neurons. It should have read L4 and L2/3 neurons. The error has been corrected for the print, PDF and HTML versions of this article.
References
Priebe, N.J. & Ferster, D. Mechanisms of neuronal computation in mammalian visual cortex. Neuron 75, 194–208 (2012).
Reid, R.C. From functional architecture to functional connectomics. Neuron 75, 209–217 (2012).
Callaway, E.M. Local circuits in primary visual cortex of the macaque monkey. Annu. Rev. Neurosci. 21, 47–74 (1998).
Douglas, R.J. & Martin, K.A. Neuronal circuits of the neocortex. Annu. Rev. Neurosci. 27, 419–451 (2004).
Harris, K.D. & Mrsic-Flogel, T.D. Cortical connectivity and sensory coding. Nature 503, 51–58 (2013).
Sun, W., Tan, Z., Mensh, B.D. & Ji, N. Thalamus provides layer 4 of primary visual cortex with orientation- and direction-tuned inputs. Nat. Neurosci. 19, 308–315 (2016).
Hubel, D.H. & Wiesel, T.N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J. Physiol. (Lond.) 160, 106–154 (1962).
Ferster, D., Chung, S. & Wheat, H. Orientation selectivity of thalamic input to simple cells of cat visual cortex. Nature 380, 249–252 (1996).
Chung, S. & Ferster, D. Strength and orientation tuning of the thalamic input to simple cells revealed by electrically evoked cortical suppression. Neuron 20, 1177–1189 (1998).
Lien, A.D. & Scanziani, M. Tuned thalamic excitation is amplified by visual cortical circuits. Nat. Neurosci. 16, 1315–1323 (2013).
Li, L.Y., Li, Y.T., Zhou, M., Tao, H.W. & Zhang, L.I. Intracortical multiplication of thalamocortical signals in mouse auditory cortex. Nat. Neurosci. 16, 1179–1181 (2013).
Li, Y.T., Ibrahim, L.A., Liu, B.H., Zhang, L.I. & Tao, H.W. Linear transformation of thalamocortical input by intracortical excitation. Nat. Neurosci. 16, 1324–1330 (2013).
Douglas, R.J., Koch, C., Mahowald, M., Martin, K.A.C. & Suarez, H.H. Recurrent excitation in neocortical circuits. Science 269, 981–985 (1995).
Ben-Yishai, R., Bar-Or, R.L. & Sompolinsky, H. Theory of orientation tuning in visual cortex. Proc. Natl. Acad. Sci. USA 92, 3844–3848 (1995).
Somers, D.C., Nelson, S.B. & Sur, M. An emergent model of orientation selectivity in cat visual cortical simple cells. J. Neurosci. 15, 5448–5465 (1995).
Song, S., Sjöström, P.J., Reigl, M., Nelson, S. & Chklovskii, D.B. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 3, e68 (2005).
Perin, R., Berger, T.K. & Markram, H. A synaptic organizing principle for cortical neuronal groups. Proc. Natl. Acad. Sci. USA 108, 5419–5424 (2011).
Yoshimura, Y., Dantzker, J.L.M. & Callaway, E.M. Excitatory cortical neurons form fine-scale functional networks. Nature 433, 868–873 (2005).
Peters, A. & Feldman, M.L. The projection of the lateral geniculate nucleus to area 17 of the rat cerebral cortex. IV. Terminations upon spiny dendrites. J. Neurocytol. 6, 669–689 (1977).
Antonini, A., Fagiolini, M. & Stryker, M.P. Anatomical correlates of functional plasticity in mouse visual cortex. J. Neurosci. 19, 4388–4406 (1999).
Ji, X.-Y. et al. Thalamocortical innervation pattern in mouse auditory and visual cortex: laminar and cell-type specificity. Cereb. Cortex 26, 2612–2625 (2016).
Petreanu, L., Huber, D., Sobczyk, A. & Svoboda, K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat. Neurosci. 10, 663–668 (2007).
Petreanu, L., Mao, T., Sternson, S.M. & Svoboda, K. The subcellular organization of neocortical excitatory connections. Nature 457, 1142–1145 (2009).
Cruikshank, S.J., Urabe, H., Nurmikko, A.V. & Connors, B.W. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron 65, 230–245 (2010).
Cruikshank, S.J., Lewis, T.J. & Connors, B.W. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat. Neurosci. 10, 462–468 (2007).
Gabernet, L., Jadhav, S.P., Feldman, D.E., Carandini, M. & Scanziani, M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron 48, 315–327 (2005).
Inoue, T. & Imoto, K. Feedforward inhibitory connections from multiple thalamic cells to multiple regular-spiking cells in layer 4 of the somatosensory cortex. J. Neurophysiol. 96, 1746–1754 (2006).
Blitz, D.M. & Regehr, W.G. Timing and specificity of feed-forward inhibition within the LGN. Neuron 45, 917–928 (2005).
Kampa, B.M., Letzkus, J.J. & Stuart, G.J. Cortical feed-forward networks for binding different streams of sensory information. Nat. Neurosci. 9, 1472–1473 (2006).
Yoshimura, Y. & Callaway, E.M. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat. Neurosci. 8, 1552–1559 (2005).
Stratford, K.J., Tarczy-Hornoch, K., Martin, K.A.C., Bannister, N.J. & Jack, J.J.B. Excitatory synaptic inputs to spiny stellate cells in cat visual cortex. Nature 382, 258–261 (1996).
Gil, Z., Connors, B.W. & Amitai, Y. Efficacy of thalamocortical and intracortical synaptic connections: quanta, innervation, and reliability. Neuron 23, 385–397 (1999).
Kloc, M. & Maffei, A. Target-specific properties of thalamocortical synapses onto layer 4 of mouse primary visual cortex. J. Neurosci. 34, 15455–15465 (2014).
Jin, J., Wang, Y., Swadlow, H.A. & Alonso, J.M. Population receptive fields of ON and OFF thalamic inputs to an orientation column in visual cortex. Nat. Neurosci. 14, 232–238 (2011).
Reid, R.C. & Alonso, J.M. Specificity of monosynaptic connections from thalamus to visual cortex. Nature 378, 281–284 (1995).
Ko, H. et al. Functional specificity of local synaptic connections in neocortical networks. Nature 473, 87–91 (2011).
Cossell, L. et al. Functional organization of excitatory synaptic strength in primary visual cortex. Nature 518, 399–403 (2015).
Wertz, A. et al. Presynaptic networks. Single-cell-initiated monosynaptic tracing reveals layer-specific cortical network modules. Science 349, 70–74 (2015).
Constantinople, C.M. & Bruno, R.M. Deep cortical layers are activated directly by thalamus. Science 340, 1591–1594 (2013).
Lefort, S., Tomm, C., Floyd Sarria, J.-C. & Petersen, C.C.H. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron 61, 301–316 (2009).
Binzegger, T., Douglas, R.J. & Martin, K.A.C. A quantitative map of the circuit of cat primary visual cortex. J. Neurosci. 24, 8441–8453 (2004).
Shepherd, G.M.G., Pologruto, T.A. & Svoboda, K. Circuit analysis of experience-dependent plasticity in the developing rat barrel cortex. Neuron 38, 277–289 (2003).
López-Bendito, G. & Molnár, Z. Thalamocortical development: how are we going to get there? Nat. Rev. Neurosci. 4, 276–289 (2003).
Yu, Y.-C., Bultje, R.S., Wang, X. & Shi, S.-H. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature 458, 501–504 (2009).
Ko, H. et al. The emergence of functional microcircuits in visual cortex. Nature 496, 96–100 (2013).
Hertz, J., Krogh, A. & Palmer, R. Introduction to the Theory of Neural Computation (Addison Wesley, 1991).
Petersen, C.C.H. & Crochet, S. Synaptic computation and sensory processing in neocortical layer 2/3. Neuron 78, 28–48 (2013).
Suter, B.A. et al. Ephus: multipurpose data acquisition software for neuroscience experiments. Front. Neural Circuits 4, 100 (2010).
Acknowledgements
We thank G. Shepherd, S. Peron, B. Atallah, H. Young, M. Fridman, A. Renart, S. Druckmann, T. Marques and C. Machens for comments on the manuscript. This work was supported by fellowships from Fundação para a Ciência e a Tecnologia to N.A.M. and J.B., a Marie Curie (PCIG12-GA-2012-334353) grant and a Human Frontier Science Program grant to L.P. and by the Champalimaud Foundation.
Author information
Authors and Affiliations
Contributions
N.A.M. and L.P. designed the study. L.P. built the experimental setup. N.A.M. performed the experiments. J.B. developed the model. N.A.M. and L.P. analyzed the data. N.A.M and L.P. wrote the manuscript with input from J.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 dLGN inputs innervate L5 pyramidal neurons in V1.
Top. Average sCRACM maps aligned by pia position (white triangles, soma position). Bottom. Average sCRACM maps aligned by soma. Only neurons receiving significant dLGN inputs (3 out of 11 neurons) are plotted.
Supplementary Figure 2 Laminar, morphological and electrophysiological differences between L4 and L2/3 pyramidal neurons.
(a) Laminar position of paired recorded neurons in L4, L2/3 or L4 and L2/3. (b) Neuronal dendritic morphology reconstructions form L2/3 (top) or L4 (bottom) neurons. (c) Input/output curves from L2/3 or L4 neurons. (d) Intrinsic properties of L4 and L2/3 recorded neurons. Vm: P = 3.02 × 10−7, t-test (t172 = −5.33). Cm: P = 1.24 × 10−26, t-test (t132 = −13.5). Rm: P = 0.1312, t-test (t132 = 1.52). Vm: membrane resting potential. Cm: membrane capacitance. Rm: membrane resistance.
Supplementary Figure 3 Example experiments from connected and nonconnected pairs of neurons in V1.
(a,e,i,m) Local connectivity test. Presynaptic spiking elicited for each neuron (top) and the traces simultaneously recorded in the other neuron (bottom). Traces are the mean of 100 repetitions. (b,f,j,n) Brightfield image of the brain slice showing the recording pipettes and the photostimulation grid. (c,g,k,o) Maps of mean eEPSC charge (top) and response probability (bottom) across photostimulated locations in b,f,j,n respectively for each neuron in the pair. White triangles, soma position. (d,h,l,p) Top, map of mean number of inputs recruited for the pair at each photostimulated location in the grid (average of top panels in c,g,k,o respectively). Mean number of inputs is calculated as the mean eEPSC charge for the pair / TC unitary input size. White circles indicate locations with correlated eEPSCs. Bottom, fraction of correlated locations as function of mean number of inputs. Bin size, 1 input. Shaded area, range used for analyses in Fig. 4 and Fig. 5. Pixels with values within that range are marked with black dots in top panel.
Supplementary Figure 4 Photostimulation fails to evoke suprathreshold responses in V1 neurons.
(a) Example photostimulation experiment in current-clamp showing subthreshold membrane potential depolarizations evoked at each location for a pair of neurons (circles). (b) Summary table for depolarizations recorded in current-clamp from L2/3 and L4 neurons.
Supplementary Figure 5 The fraction of correlated locations increases with the number of recruited inputs.
(a) Fraction of locations with correlated inputs as a function of the mean number of inputs recruited from all photostimulated locations and all the pairs in L4, L2/3 or L4→L2/3 (connected and not connected pairs pooled together). Bin size 1 input. Exact fraction of correlated locations in each bin are shown for L4, L2/3 and L4→L2/3 pairs. First 4 bins are the same data as in Fig. 4 b, e and Fig. 5 d. (b) Mean number of inputs versus distance of the photostimulated locations to each L4 pair (center of both somata). Bin size, 100 μm. The number of locations in each bin is shown. (c) Fraction of correlated locations as function of distance to the pair for all L4 pairs. Bin size, 100 μm. The fraction of correlated locations in each bin is shown. The number of inputs recruited is larger in perisomatic areas resulting in a larger fraction of correlated locations close to the somata in L4 pairs.
Supplementary Figure 6 Connection strength is a weak predictor of shared input.
Relationship between the fraction of correlated locations and the connection strength for L4 (a), L2/3 (b) and L4→L2/3 (c) pairs. Gray line, linear regression fit.
Supplementary Figure 7 Intersomatic distances of the recorded pairs.
Lateral (a,b,c), vertical (d,e,f) and total distance (g,h,i) for the connected and not connected pairs in each group. L4→L2/3 distances are shown for all pairs (left) or only the pairs within 60 μm of lateral distance (right). P value for Wilcoxon rank sum test between groups is shown in each panel. Horizontal lines, mean. (j,k,l) Fraction of correlated locations as function of lateral distance for L4, L2/3 and L4→L2/3 pairs. Gray line, linear regression fit. Filled dots, bidirectionally connected pairs. For L4→L2/3 pairs there was a significant effect of horizontal distance on the fraction of correlated locations. However, as there was only one connected pair at distances > 60 μm, the effect could be due to connectivity and not distance. To disambiguate this we compared correlations only for pairs < 60 μm apart. While lateral displacement was very similar across the two groups (panel c, right), the fraction of correlated locations was still larger in the connected group (inset in panel l, fraction of correlated locations for individual vertical pairs within 60 μm of lateral distance; P < 0.02 Wilcoxon rank sum test).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 1475 kb)
Rights and permissions
About this article
Cite this article
Morgenstern, N., Bourg, J. & Petreanu, L. Multilaminar networks of cortical neurons integrate common inputs from sensory thalamus. Nat Neurosci 19, 1034–1040 (2016). https://doi.org/10.1038/nn.4339
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4339
This article is cited by
-
Haploinsufficiency of Shank3 increases the orientation selectivity of V1 neurons
Scientific Reports (2022)
-
Auditory input enhances somatosensory encoding and tactile goal-directed behavior
Nature Communications (2021)
-
Untangling the cortico-thalamo-cortical loop: cellular pieces of a knotty circuit puzzle
Nature Reviews Neuroscience (2021)
-
Survey of spiking in the mouse visual system reveals functional hierarchy
Nature (2021)
-
NeuroPath2Path: Classification and elastic morphing between neuronal arbors using path-wise similarity
Neuroinformatics (2020)